Strategies to Increase Cervical Cancer Screening With Mailed Human Papillomavirus Self-Sampling Kits: A Randomized Clinical Trial
- PMID: 38015219
- PMCID: PMC10685881
- DOI: 10.1001/jama.2023.21471
Strategies to Increase Cervical Cancer Screening With Mailed Human Papillomavirus Self-Sampling Kits: A Randomized Clinical Trial
Abstract
Importance: Optimal strategies for increasing cervical cancer screening may differ by patient screening history and health care setting. Mailing human papillomavirus (HPV) self-sampling kits to individuals who are overdue for screening increases adherence; however, offering self-sampling kits to screening-adherent individuals has not been evaluated in the US.
Objective: To evaluate the effectiveness of direct-mail and opt-in approaches for offering HPV self-sampling kits to individuals by cervical cancer screening history (screening-adherent and currently due, overdue, or unknown).
Design, setting, and participants: Randomized clinical trial conducted in Kaiser Permanente Washington, a US integrated health care delivery system. Individuals aged 30 to 64 years with female sex, a primary care clinician, and no hysterectomy were identified through electronic health records (EHRs) and enrolled between November 20, 2020, and January 28, 2022, with follow-up through July 29, 2022.
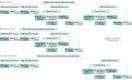
Interventions: Individuals stratified as due (eg, at the time of randomization, these individuals have been previously screened and are due for their next screening in ≤3 months) were randomized to receive usual care (patient reminders and clinician EHR alerts [n = 3671]), education (usual care plus educational materials about screening [n = 3960]), direct mail (usual care plus educational materials and a mailed self-sampling kit [n = 1482]), or to opt in (usual care plus educational materials and the option to request a kit [n = 3956]). Individuals who were overdue for screening were randomized to receive usual care (n = 5488), education (n = 1408), or direct mail (n = 1415). Individuals with unknown history for screening were randomized to receive usual care (n = 2983), education (n = 3486), or to opt in (n = 3506).
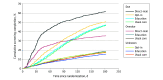
Main outcomes and measures: The primary outcome was screening completion within 6 months. Primary analyses compared direct-mail or opt-in participants with individuals randomized to the education group.
Results: The intention-to-treat analyses included 31 355 randomized individuals (mean [SD] age, 45.9 [10.4] years). Among those who were due for screening, compared with receiving education alone (1885 [47.6%]), screening completion was 14.1% (95% CI, 11.2%-16.9%) higher in the direct-mail group (914 [61.7%]) and 3.5% (95% CI, 1.2%-5.7%) higher in the opt-in group (2020 [51.1%]). Among individuals who were overdue, screening completion was 16.9% (95% CI, 13.8%-20.0%) higher in the direct-mail group (505 [35.7%]) compared with education alone (264 [18.8%]). Among those with unknown history, screening was 2.2% (95% CI, 0.5%-3.9%) higher in the opt-in group (634 [18.1%]) compared with education alone (555 [15.9%]).
Conclusions and relevance: Within a US health care system, direct-mail self-sampling increased cervical cancer screening by more than 14% in individuals who were due or overdue for cervical cancer screening. The opt-in approach minimally increased screening. To increase screening adherence, systems implementing HPV self-sampling should prioritize direct-mail outreach for individuals who are due or overdue for screening. For individuals with unknown screening history, testing alternative outreach approaches and additional efforts to document screening history are warranted.
Trial registration: ClinicalTrials.gov Identifier: NCT04679675.
Conflict of interest statement
Figures

Comment in
-
For due or overdue cervical cancer screening, direct or opt-in mailing of HPV self-sampling increased screening at 6 mo vs. education.Ann Intern Med. 2024 Apr;177(4):JC46. doi: 10.7326/J24-0019. Epub 2024 Apr 2. Ann Intern Med. 2024. PMID: 38560899
References
-
- Centers for Disease Control and Prevention . Cancers associated with human papillomavirus, United States—2012–2016. Published May 4, 2022. Accessed November 5, 2023. https://www.cdc.gov/cancer/uscs/about/data-briefs/no10-hpv-assoc-cancers...
-
- Suk R, Hong YR, Rajan SS, Xie Z, Zhu Y, Spencer JC. Assessment of US Preventive Services Task Force Guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5(1):e2143582. doi: 10.1001/jamanetworkopen.2021.43582 - DOI - PMC - PubMed

