Toripalimab Plus Chemotherapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: The JUPITER-02 Randomized Clinical Trial
- PMID: 38015220
- PMCID: PMC10685882
- DOI: 10.1001/jama.2023.20181
Toripalimab Plus Chemotherapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: The JUPITER-02 Randomized Clinical Trial
Abstract
Importance: There are currently no therapies approved by the US Food and Drug Administration for nasopharyngeal carcinoma (NPC). Gemcitabine-cisplatin is the current standard of care for the first-line treatment of recurrent or metastatic NPC (RM-NPC).
Objective: To determine whether toripalimab in combination with gemcitabine-cisplatin will significantly improve progression-free survival and overall survival as first-line treatment for RM-NPC, compared with gemcitabine-cisplatin alone.
Design, setting, and participants: JUPITER-02 is an international, multicenter, randomized, double-blind phase 3 study conducted in NPC-endemic regions, including mainland China, Taiwan, and Singapore. From November 10, 2018, to October 20, 2019, 289 patients with RM-NPC with no prior systemic chemotherapy in the RM setting were enrolled from 35 participating centers.
Interventions: Patients were randomized (1:1) to receive toripalimab (240 mg [n = 146]) or placebo (n = 143) in combination with gemcitabine-cisplatin for up to 6 cycles, followed by maintenance with toripalimab or placebo until disease progression, intolerable toxicity, or completion of 2 years of treatment.
Main outcome: Progression-free survival as assessed by a blinded independent central review. Secondary end points included objective response rate, overall survival, progression-free survival assessed by investigator, duration of response, and safety.
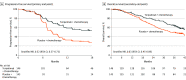
Results: Among the 289 patients enrolled (median age, 46 [IQR, 38-53 years; 17% female), at the final progression-free survival analysis, toripalimab treatment had a significantly longer progression-free survival than placebo (median, 21.4 vs 8.2 months; HR, 0.52 [95% CI, 0.37-0.73]). With a median survival follow-up of 36.0 months, a significant improvement in overall survival was identified with toripalimab over placebo (hazard ratio [HR], 0.63 [95% CI, 0.45-0.89]; 2-sided P = .008). The median overall survival was not reached in the toripalimab group, while it was 33.7 months in the placebo group. A consistent effect on overall survival, favoring toripalimab, was found in subgroups with high and low PD-L1 (programmed death-ligand 1) expression. The incidence of all adverse events, grade 3 or greater adverse events, and fatal adverse events were similar between the 2 groups. However, adverse events leading to discontinuation of toripalimab or placebo (11.6% vs 4.9%), immune-related adverse events (54.1% vs 21.7%), and grade 3 or greater immune-related adverse events (9.6% vs 1.4%) were more frequent in the toripalimab group.
Conclusions and relevance: The addition of toripalimab to chemotherapy as first-line treatment for RM-NPC provided statistically significant and clinically meaningful progression-free survival and overall survival benefits compared with chemotherapy alone, with a manageable safety profile. These findings support the use of toripalimab plus gemcitabine-cisplatin as the new standard of care for this patient population.
Trial registration: ClinicalTrials.gov Identifier: NCT03581786.
Conflict of interest statement
Figures
Comment in
-
Immunotherapy for Nasopharyngeal Carcinoma: The Earlier the Better.JAMA. 2023 Nov 28;330(20):1954-1955. doi: 10.1001/jama.2023.22465. JAMA. 2023. PMID: 38015229 No abstract available.
-
Immunotherapeutics in nasopharyngeal carcinoma: a relentless CONTINUUM of success.Lancet. 2024 Jun 22;403(10445):2667-2669. doi: 10.1016/S0140-6736(24)00810-9. Epub 2024 May 30. Lancet. 2024. PMID: 38824939 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

