Barriers and facilitators to the implementation of a new European eHealth solution (SurPass v2.0): the PanCareSurPass Open Space study
- PMID: 38015382
- PMCID: PMC11926050
- DOI: 10.1007/s11764-023-01498-8
Barriers and facilitators to the implementation of a new European eHealth solution (SurPass v2.0): the PanCareSurPass Open Space study
Erratum in
-
Correction: Barriers and facilitators to the implementation of a new European eHealth solution (SurPass v2.0): the PanCareSurPass Open Space study.J Cancer Surviv. 2025 Jun;19(3):1111. doi: 10.1007/s11764-024-01547-w. J Cancer Surviv. 2025. PMID: 38466480 Free PMC article. No abstract available.
Abstract
Purpose: To identify barriers and facilitators for implementing the Survivorship Passport (SurPass) v2.0 in six long-term follow-up (LTFU) care centres in Europe.
Methods: Stakeholders including childhood cancer survivors (CCSs), healthcare providers (HCPs), managers, information and technology (IT) specialists, and others, participated in six online Open Space meetings. Topics related to Care, Ethical, Legal, Social, Economic, and Information & IT-related aspects of implementing SurPass were evaluated.
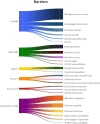
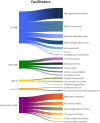
Results: The study identified 115 barriers and 159 facilitators. The main barriers included the lack of standardised LTFU care in centres and network cooperation, uncertainty about SurPass accessibility, and uncertainty about how to integrate SurPass into electronic health information systems. The main facilitators included standardised and coordinated LTFU care in centres, allowing CCSs to conceal sensitive information in SurPass and (semi)automatic data transfer and filing.
Conclusions: Key barriers to SurPass implementation were identified in the areas of care, ethical considerations, and information & IT. To address these barriers and facilitate the implementation on SurPass, we have formulated 27 recommendations. Key recommendations include using the internationally developed protocols and guidelines to implement LTFU care, making clear decisions about which parties have access to SurPass data in accordance with CCSs, and facilitating (semi)automated data transfer and filing using Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR).
Implications for cancer survivors: The findings of this study can help to implement SurPass and to ensure that cancer survivors receive high-quality LTFU care with access to the necessary information to manage their health effectively.
Keywords: Long-term follow-up care; Open Space Technology; Paediatric oncology; SurPass; Survivorship Passport; Survivorship care; eHealth.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Ethical review board approval was required and obtained in Spain only (Comité de Ética de la Investigación con medicamentos—Hospital Universitario y Politécnico La Fe). This study was performed in line with the principles of the Declaration of Helsinki. Consent to participate: Written informed consent was obtained from all Spanish participants included in this study. Consent for publication: No personal data was collected during this study. Participants were informed that by participating in this study, they consent to publication of their input during the Open Space meetings. Competing interests: The authors declare no competing interests.
Figures
References
-
- World Health Organization. Childhood cancer. https://www.who.int/news-room/fact-sheets/detail/cancer-in-children. Accessed 13 Dec 2021.
-
- SIOP Europe: the European society for paediatric oncology. The SIOPE strategic plan a European cancer plan for children and adolescents. 2015. https://worldspanmedia.s3-eu-west-1.amazonaws.com/media/siope/PDF/the-si.... Accessed August 30, 2022.
-
- Francisci S, Guzzinati S, Dal Maso L, Sacerdote C, Buzzoni C, Gigli A. An estimate of the number of people in Italy living after childhood cancer. Int J Cancer. 2017;140(11):2444–50. 10.1002/ijc.30665. - PubMed
-
- Geenen MM, Cardous-Ubbink MC, Kremer LC, Van den Bos C, Van der Pal HJH, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705. 10.1001/jama.297.24.2705. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

