A Placebo-Controlled Trial of Percutaneous Coronary Intervention for Stable Angina
- PMID: 38015442
- PMCID: PMC7615400
- DOI: 10.1056/NEJMoa2310610
A Placebo-Controlled Trial of Percutaneous Coronary Intervention for Stable Angina
Abstract
Background: Percutaneous coronary intervention (PCI) is frequently performed to reduce the symptoms of stable angina. Whether PCI relieves angina more than a placebo procedure in patients who are not receiving antianginal medication remains unknown.
Methods: We conducted a double-blind, randomized, placebo-controlled trial of PCI in patients with stable angina. Patients stopped all antianginal medications and underwent a 2-week symptom assessment phase before randomization. Patients were then randomly assigned in a 1:1 ratio to undergo PCI or a placebo procedure and were followed for 12 weeks. The primary end point was the angina symptom score, which was calculated daily on the basis of the number of angina episodes that occurred on a given day, the number of antianginal medications prescribed on that day, and clinical events, including the occurrence of unblinding owing to unacceptable angina or acute coronary syndrome or death. Scores range from 0 to 79, with higher scores indicating worse health status with respect to angina.
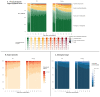
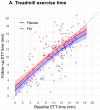
Results: A total of 301 patients underwent randomization: 151 to the PCI group and 150 to the placebo group. The mean (±SD) age was 64±9 years, and 79% were men. Ischemia was present in one cardiac territory in 242 patients (80%), in two territories in 52 patients (17%), and in three territories in 7 patients (2%). In the target vessels, the median fractional flow reserve was 0.63 (interquartile range, 0.49 to 0.75), and the median instantaneous wave-free ratio was 0.78 (interquartile range, 0.55 to 0.87). At the 12-week follow-up, the mean angina symptom score was 2.9 in the PCI group and 5.6 in the placebo group (odds ratio, 2.21; 95% confidence interval, 1.41 to 3.47; P<0.001). One patient in the placebo group had unacceptable angina leading to unblinding. Acute coronary syndromes occurred in 4 patients in the PCI group and in 6 patients in the placebo group.
Conclusions: Among patients with stable angina who were receiving little or no antianginal medication and had objective evidence of ischemia, PCI resulted in a lower angina symptom score than a placebo procedure, indicating a better health status with respect to angina. (Funded by the National Institute for Health and Care Research Imperial Biomedical Research Centre and others; ORBITA-2 ClinicalTrials.gov number, NCT03742050.).
Copyright © 2023 Massachusetts Medical Society.
Figures
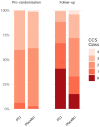
Comment in
-
PCI for stable angina.Nat Rev Cardiol. 2024 Feb;21(2):71. doi: 10.1038/s41569-023-00967-y. Nat Rev Cardiol. 2024. PMID: 38036671 No abstract available.
-
In adults with stable angina, PCI vs. a placebo procedure reduced angina symptoms at 12 wk.Ann Intern Med. 2024 Mar;177(3):JC28. doi: 10.7326/J24-0009. Epub 2024 Mar 5. Ann Intern Med. 2024. PMID: 38437691
-
A Placebo-Controlled Trial of PCI for Stable Angina.N Engl J Med. 2024 Mar 21;390(12):1149-1150. doi: 10.1056/NEJMc2400751. N Engl J Med. 2024. PMID: 38507760 No abstract available.
-
A Placebo-Controlled Trial of PCI for Stable Angina.N Engl J Med. 2024 Mar 21;390(12):1150. doi: 10.1056/NEJMc2400751. N Engl J Med. 2024. PMID: 38507761 Free PMC article. No abstract available.
-
A Placebo-Controlled Trial of PCI for Stable Angina.N Engl J Med. 2024 Mar 21;390(12):1150-1151. doi: 10.1056/NEJMc2400751. N Engl J Med. 2024. PMID: 38507762 No abstract available.
-
A Placebo-Controlled Trial of PCI for Stable Angina. Reply.N Engl J Med. 2024 Mar 21;390(12):1151-1152. doi: 10.1056/NEJMc2400751. N Engl J Med. 2024. PMID: 38507763 No abstract available.
-
A Placebo-Controlled Trial of PCI for Stable Angina. Reply.N Engl J Med. 2024 Mar 21;390(12):10.1056/NEJMc2400751#sa5. doi: 10.1056/NEJMc2400751. N Engl J Med. 2024. PMID: 38507764 No abstract available.
References
-
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. - PubMed
-
- Parisi AF, Folland ED, Hartigan P. A Comparison of Angioplasty with Medical Therapy in the Treatment of Single-Vessel Coronary Artery Disease. N Engl J Med. 1992;326:10–6. - PubMed
-
- De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- FS/CRA/22/23036/BHF_/British Heart Foundation/United Kingdom
- MR/S021108/1/MRC_/Medical Research Council/United Kingdom
- FS/ICRF/22/26051/BHF_/British Heart Foundation/United Kingdom
- FS/ICRF/22/26039/BHF_/British Heart Foundation/United Kingdom
- MR/W000520/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
