PD-1 Impairs CD8+ T Cell Granzyme B Production in Aged Mice during Acute Viral Respiratory Infection
- PMID: 38015461
- PMCID: PMC10696419
- DOI: 10.4049/immunohorizons.2300094
PD-1 Impairs CD8+ T Cell Granzyme B Production in Aged Mice during Acute Viral Respiratory Infection
Abstract
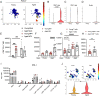
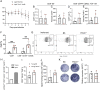
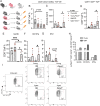
CD8+ T cell dysfunction contributes to severe respiratory viral infection outcomes in older adults. CD8+ T cells are the primary cell type responsible for viral clearance. With increasing age, CD8+ T cell function declines in conjunction with an accumulation of cytotoxic tissue-resident memory (TRM) CD8+ T cells. We sought to elucidate the role of PD-1 signaling on aged CD8+ T cell function and accumulation of CD8+ TRM cells during acute viral respiratory tract infection, given the importance of PD-1 regulating CD8+ T cells during acute and chronic infections. PD-1 blockade or genetic ablation in aged mice yielded improved CD8+ T cell granzyme B production comparable to that in young mice during human metapneumovirus and influenza viral infections. Syngeneic transplant and adoptive transfer strategies revealed that improved granzyme B production in aged Pdcd1-/- CD8+ T cells was primarily cell intrinsic because aged wild-type CD8+ T cells did not have increased granzyme B production when transplanted into a young host. PD-1 signaling promoted accumulation of cytotoxic CD8+ TRM cells in aged mice. PD-1 blockade of aged mice during rechallenge infection resulted in improved clinical outcomes that paralleled reduced accumulation of CD8+ TRM cells. These findings suggest that PD-1 signaling impaired CD8+ T cell granzyme B production and contributed to CD8+ TRM cell accumulation in the aged lung. These findings have implications for future research investigating PD-1 checkpoint inhibitors as a potential therapeutic option for elderly patients with severe respiratory viral infections.
Copyright © 2023 The Authors.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




Similar articles
-
PD-1 signaling in neonates restrains CD8+ T cell function and protects against respiratory viral immunopathology.Mucosal Immunol. 2024 Jun;17(3):476-490. doi: 10.1016/j.mucimm.2023.12.004. Epub 2024 Jan 3. Mucosal Immunol. 2024. PMID: 38176655 Free PMC article.
-
Tertiary Lymphoid Structure-Associated B Cells Enhance CXCL13+CD103+CD8+ Tissue-Resident Memory T-Cell Response to Programmed Cell Death Protein 1 Blockade in Cancer Immunotherapy.Gastroenterology. 2024 Jun;166(6):1069-1084. doi: 10.1053/j.gastro.2023.10.022. Epub 2023 Oct 29. Gastroenterology. 2024. PMID: 38445519
-
Programmed death-1 impairs secondary effector lung CD8⁺ T cells during respiratory virus reinfection.J Immunol. 2014 Nov 15;193(10):5108-17. doi: 10.4049/jimmunol.1302208. Epub 2014 Oct 22. J Immunol. 2014. PMID: 25339663 Free PMC article.
-
Viral acute lower respiratory infections impair CD8+ T cells through PD-1.J Clin Invest. 2012 Aug;122(8):2967-82. doi: 10.1172/JCI62860. Epub 2012 Jul 17. J Clin Invest. 2012. PMID: 22797302 Free PMC article.
-
Persistence in Temporary Lung Niches: A Survival Strategy of Lung-Resident Memory CD8+ T Cells.Viral Immunol. 2017 Jul/Aug;30(6):438-450. doi: 10.1089/vim.2017.0016. Epub 2017 Apr 18. Viral Immunol. 2017. PMID: 28418771 Free PMC article. Review.
Cited by
-
In vivo labeling reveals that degranulation is increased under supraphysiological TCR stimulation, but not infection, in CD8+ T cells from old mice.Geroscience. 2025 Jun 6. doi: 10.1007/s11357-025-01723-5. Online ahead of print. Geroscience. 2025. PMID: 40478430
-
Alarm Functions of PD-1+ Brain-Resident Memory T Cells.J Immunol. 2024 Dec 1;213(11):1585-1594. doi: 10.4049/jimmunol.2400295. J Immunol. 2024. PMID: 39413000
References
-
- Falsey, A. R., Erdman D., Anderson L. J., Walsh E. E.. 2003. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187: 785–790. - PubMed
-
- van den Hoogen, B. G., Osterhaus D. M., Fouchier R. A.. 2004. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr. Infect. Dis. J. 23(1 Suppl): S25–S32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

