TIMP2 ameliorates blood-brain barrier disruption in traumatic brain injury by inhibiting Src-dependent VE-cadherin internalization
- PMID: 38015626
- PMCID: PMC10849766
- DOI: 10.1172/JCI164199
TIMP2 ameliorates blood-brain barrier disruption in traumatic brain injury by inhibiting Src-dependent VE-cadherin internalization
Abstract
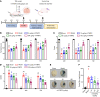
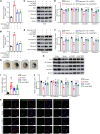
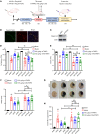
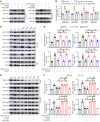
Blood-brain barrier (BBB) disruption is a serious pathological consequence of traumatic brain injury (TBI), for which there are limited therapeutic strategies. Tissue inhibitor of metalloproteinase-2 (TIMP2), a molecule with dual functions of inhibiting MMP activity and displaying cytokine-like activity through receptor binding, has been reported to inhibit VEGF-induced vascular hyperpermeability. Here, we investigate the ability of TIMP2 to ameliorate BBB disruption in TBI and the underlying molecular mechanisms. Both TIMP2 and AlaTIMP2, a TIMP2 mutant without MMP-inhibiting activity, attenuated neurological deficits and BBB leakage in TBI mice; they also inhibited junctional protein degradation and translocation to reduce paracellular permeability in human brain microvascular endothelial cells (ECs) exposed to hypoxic plus inflammatory insult. Mechanistic studies revealed that TIMP2 interacted with α3β1 integrin on ECs, inhibiting Src activation-dependent VE-cadherin phosphorylation, VE-cadherin/catenin complex destabilization, and subsequent VE-cadherin internalization. Notably, localization of VE-cadherin on the membrane was critical for TIMP2-mediated EC barrier integrity. Furthermore, TIMP2-mediated increased membrane localization of VE-cadherin enhanced the level of active Rac1, thereby inhibiting stress fiber formation. All together, our studies have identified an MMP-independent mechanism by which TIMP2 regulates EC barrier integrity after TBI. TIMP2 may be a therapeutic agent for TBI and other neurological disorders involving BBB breakdown.
Keywords: Molecular biology; Neurodegeneration; Neuroscience; Vascular Biology.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

