Age-related loss of Notch3 underlies brain vascular contractility deficiencies, glymphatic dysfunction, and neurodegeneration in mice
- PMID: 38015629
- PMCID: PMC10786701
- DOI: 10.1172/JCI166134
Age-related loss of Notch3 underlies brain vascular contractility deficiencies, glymphatic dysfunction, and neurodegeneration in mice
Abstract
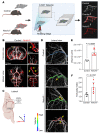
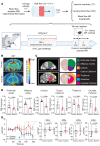
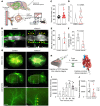
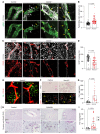
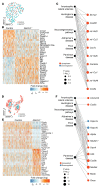
Vascular aging affects multiple organ systems, including the brain, where it can lead to vascular dementia. However, a concrete understanding of how aging specifically affects the brain vasculature, along with molecular readouts, remains vastly incomplete. Here, we demonstrate that aging is associated with a marked decline in Notch3 signaling in both murine and human brain vessels. To clarify the consequences of Notch3 loss in the brain vasculature, we used single-cell transcriptomics and found that Notch3 inactivation alters regulation of calcium and contractile function and promotes a notable increase in extracellular matrix. These alterations adversely impact vascular reactivity, manifesting as dilation, tortuosity, microaneurysms, and decreased cerebral blood flow, as observed by MRI. Combined, these vascular impairments hinder glymphatic flow and result in buildup of glycosaminoglycans within the brain parenchyma. Remarkably, this phenomenon mirrors a key pathological feature found in brains of patients with CADASIL, a hereditary vascular dementia associated with NOTCH3 missense mutations. Additionally, single-cell RNA sequencing of the neuronal compartment in aging Notch3-null mice unveiled patterns reminiscent of those observed in neurodegenerative diseases. These findings offer direct evidence that age-related NOTCH3 deficiencies trigger a progressive decline in vascular function, subsequently affecting glymphatic flow and culminating in neurodegeneration.
Keywords: Cardiovascular disease; Dementia; Neurological disorders; Neuroscience; Vascular Biology.
Conflict of interest statement
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

