RAB7 deficiency impairs pulmonary artery endothelial function and promotes pulmonary hypertension
- PMID: 38015641
- PMCID: PMC10836802
- DOI: 10.1172/JCI169441
RAB7 deficiency impairs pulmonary artery endothelial function and promotes pulmonary hypertension
Abstract
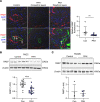
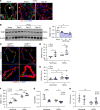
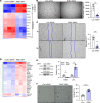
Pulmonary arterial hypertension (PAH) is a devastating and progressive disease with limited treatment options. Endothelial dysfunction plays a central role in the development and progression of PAH, yet the underlying mechanisms are incompletely understood. The endosome-lysosome system is important to maintain cellular health, and the small GTPase RAB7 regulates many functions of this system. Here, we explored the role of RAB7 in endothelial cell (EC) function and lung vascular homeostasis. We found reduced expression of RAB7 in ECs from patients with PAH. Endothelial haploinsufficiency of RAB7 caused spontaneous pulmonary hypertension (PH) in mice. Silencing of RAB7 in ECs induced broad changes in gene expression revealed via RNA-Seq, and RAB7-silenced ECs showed impaired angiogenesis and expansion of a senescent cell fraction, combined with impaired endolysosomal trafficking and degradation, suggesting inhibition of autophagy at the predegradation level. Furthermore, mitochondrial membrane potential and oxidative phosphorylation were decreased, and glycolysis was enhanced. Treatment with the RAB7 activator ML-098 reduced established PH in rats with chronic hypoxia/SU5416. In conclusion, we demonstrate for the first time to our knowledge the fundamental impairment of EC function by loss of RAB7, causing PH, and show RAB7 activation to be a potential therapeutic strategy in a preclinical model of PH.
Keywords: Autophagy; Cellular senescence; Endothelial cells; Pulmonology.
Figures





Update of
-
RAB7 deficiency impairs pulmonary artery endothelial function and promotes pulmonary hypertension.bioRxiv [Preprint]. 2023 Feb 6:2023.02.03.526842. doi: 10.1101/2023.02.03.526842. bioRxiv. 2023. Update in: J Clin Invest. 2024 Feb 1;134(3):e169441. doi: 10.1172/JCI169441. PMID: 36778418 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

