mRNA translation in astrocytes controls hippocampal long-term synaptic plasticity and memory
- PMID: 38015848
- PMCID: PMC10710058
- DOI: 10.1073/pnas.2308671120
mRNA translation in astrocytes controls hippocampal long-term synaptic plasticity and memory
Abstract
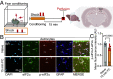
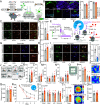
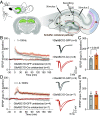
Activation of neuronal protein synthesis upon learning is critical for the formation of long-term memory. Here, we report that learning in the contextual fear conditioning paradigm engenders a decrease in eIF2α (eukaryotic translation initiation factor 2) phosphorylation in astrocytes in the hippocampal CA1 region, which promotes protein synthesis. Genetic reduction of eIF2α phosphorylation in hippocampal astrocytes enhanced contextual and spatial memory and lowered the threshold for the induction of long-lasting plasticity by modulating synaptic transmission. Thus, learning-induced dephosphorylation of eIF2α in astrocytes bolsters hippocampal synaptic plasticity and consolidation of long-term memories.
Keywords: astrocytes; integrated stress response; learning and memory; protein synthesis; synaptic plasticity.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

