Surgery with locking plate or hemiarthroplasty versus nonoperative treatment of 3-4-part proximal humerus fractures in older patients (NITEP): An open-label randomized trial
- PMID: 38015877
- PMCID: PMC10683994
- DOI: 10.1371/journal.pmed.1004308
Surgery with locking plate or hemiarthroplasty versus nonoperative treatment of 3-4-part proximal humerus fractures in older patients (NITEP): An open-label randomized trial
Abstract
Background: Proximal humerus fractures (PHFs) are common fractures, especially in older female patients. These fractures are commonly treated surgically, but the consensus on the best treatment is still lacking.
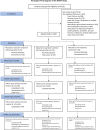
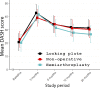
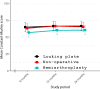
Methods and findings: The primary aim of this multicenter, randomized 3-arm superiority, open-label trial was to assess the results of nonoperative treatment and operative treatment either with locking plate (LP) or hemiarthroplasty (HA) of 3- and 4-part PHF with the primary outcome of Disabilities of the Arm, Shoulder, and Hand (DASH) at 2-year follow-up. Between February 2011 and December 2019, 160 patients 60 years and older with 3- and 4-part PHFs were randomly assigned in 1:1:1 fashion in block size of 10 to undergo nonoperative treatment (control) or operative intervention with LP or HA. In total, 54 patients were assigned to the nonoperative group, 52 to the LP group, and 54 to the HA group. Five patients assigned to the LP group were reassigned to the HA group perioperatively due to high comminution, and all of these patients had 4-part fractures. In the intention-to-treat analysis, there were 42 patients in the nonoperative group, 44 in the LP group, and 37 in the HA group. The outcome assessors were blinded to the study group. The mean DASH score at 2-year follow-up was 30.4 (standard error (SE) 3.25), 31.4 (SE 3.11), and 26.6 (SE 3.23) points for the nonoperative, LP, and HA groups, respectively. At 2 years, the between-group differences were 1.07 points (95% CI [-9.5,11.7]; p = 0.97) between nonoperative and LP, 3.78 points (95% CI [-7.0,14.6]; p = 0.69) between nonoperative and HA, and 4.84 points (95% CI [-5.7,15.4]; p = 0.53) between LP and HA. No significant differences in primary or secondary outcomes were seen in stratified age groups (60 to 70 years and 71 years and over). At 2 years, we found 30 complications (3/52, 5.8% in nonoperative; 22/49, 45% in LP; and 5/49, 10% in HA group, p = 0.0004) and 16 severe pain-related adverse events. There was a revision rate of 22% in the LP group. The limitation of the trial was that the recruitment period was longer than expected due to a high number of exclusions after the assessment of eligibility and a larger exclusion rate than anticipated toward the end of the trial. Therefore, the trial was ended prematurely.
Conclusions: In this study, no benefit was observed between operative treatment with LP or HA and nonoperative treatment in displaced 3- and 4-part PHFs in patients aged 60 years and older. Further, we observed a high rate of complications related to operative treatments.
Trial registration: ClinicalTrials.gov NCT01246167.
Copyright: © 2023 Launonen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

