Tackling late HIV diagnosis: Lessons from the UK in the COVID-19 era
- PMID: 38016099
- PMCID: PMC10908195
- DOI: 10.1177/09564624231202287
Tackling late HIV diagnosis: Lessons from the UK in the COVID-19 era
Abstract
Introduction: Late diagnosis of HIV is associated with increased morbidity and mortality, and an increased risk of non-infectious comorbidities. On a societal level, late diagnosis leads to higher treatment and healthcare costs and is a major driver of HIV transmission. Despite improvements in other areas of the HIV care pathway, late diagnosis remains an individual and public health concern globally.
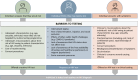
Objective: To examine the barriers to HIV testing and highlight successful strategies to improve prompt diagnosis. This review describes the prevalence of late diagnosis in the UK and discusses key factors that contribute to late diagnosis, including the effect of the COVID-19 pandemic. Late HIV diagnosis is lower in the UK than in most other European countries. In this review, pilot projects and ongoing initiatives that have reduced late diagnosis in the UK are highlighted; moreover, further strategies for improving prompt diagnosis are suggested.
Conclusions: Insufficient testing is the fundamental reason for late HIV diagnosis, with societal, systemic, and individual factors all contributing to inadequate testing. Improving access to testing, removing barriers to health-seeking behaviour, and ensuring all people with HIV indicator conditions are promptly tested are key to reducing the rates of late diagnosis globally.
Keywords: ART; Europe; HIV; diagnosis; screening.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Marta Boffito received travel grants, speaker and advisor fees, and research grants to the organisation from Janssen, Roche, ViiV, Bristol Myers Squibb, Merck Sharp & Dohme, Gilead Sciences, Mylan, Cipla, Novavax, Valneva, GSK, Atea, Moderna, Teva, AZ and Pfizer. David R Chadwick is a trustee/executive member of the British HIV Association and has recently chaired a sub-group on guidelines for Emergency Department opt-out testing using implied consent. He has also received research grants from Gilead Sciences. Samuel Kabagambe is an employee of Gilead Sciences and may own stock options in Gilead Sciences. Bakita Kasadha has previously received speaker honoraria and consultancy fees from Gilead Sciences. Cindy Elliott is an employee of Gilead Sciences and may own stock options in Gilead Sciences. Emily Boardman, and Emily Cheserem declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Sobrino-Vegas P, Moreno S, Rubio R, et al. Impact of late presentation of HIV infection on short-, mid- and long-term mortality and causes of death in a multicenter national cohort: 2004–2013. J Infect 2016; 72: 587–596. - PubMed
-
- British HIV Association . Recording and investigation of late HIV diagnoses: good practice position statement. London, UK: British HIV Association, 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

