Predicting recurrence and survival in patients with non-metastatic renal-cell carcinoma after nephrectomy: a prospective population-based study with multicenter validation
- PMID: 38016139
- PMCID: PMC10871562
- DOI: 10.1097/JS9.0000000000000935
Predicting recurrence and survival in patients with non-metastatic renal-cell carcinoma after nephrectomy: a prospective population-based study with multicenter validation
Abstract
Background: Accurate prognostication of oncological outcomes is crucial for the optimal management of patients with renal cell carcinoma (RCC) after surgery. Previous prediction models were developed mainly based on retrospective data in the Western populations, and their predicting accuracy remains limited in contemporary, prospective validation. We aimed to develop contemporary RCC prognostic models for recurrence and overall survival (OS) using prospective population-based patient cohorts and compare their performance with existing, mostly utilized ones.
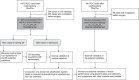
Methods: In this prospective analysis and external validation study, the development set included 11 128 consecutive patients with non-metastatic RCC treated at a tertiary urology center in China between 2006 and 2022, and the validation set included 853 patients treated at 13 medical centers in the USA between 1996 and 2013. The primary outcome was progression-free survival (PFS), and the secondary outcome was OS. Multivariable Cox regression was used for variable selection and model development. Model performance was assessed by discrimination [Harrell's C-index and time-dependent areas under the curve (AUC)] and calibration (calibration plots). Models were validated internally by bootstrapping and externally by examining their performance in the validation set. The predictive accuracy of the models was compared with validated models commonly used in clinical trial designs and with recently developed models without extensive validation.
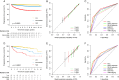
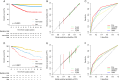
Results: Of the 11 128 patients included in the development set, 633 PFS and 588 OS events occurred over a median follow-up of 4.3 years [interquartile range (IQR) 1.7-7.8]. Six common clinicopathologic variables (tumor necrosis, size, grade, thrombus, nodal involvement, and perinephric or renal sinus fat invasion) were included in each model. The models demonstrated similar C-indices in the development set (0.790 [95% CI 0.773-0.806] for PFS and 0.793 [95% CI 0.773-0.811] for OS) and in the external validation set (0.773 [0.731-0.816] and 0.723 [0.731-0.816]). A relatively stable predictive ability of the models was observed in the development set (PFS: time-dependent AUC 0.832 at 1 year to 0.760 at 9 years; OS: 0.828 at 1 year to 0.794 at 9 years). The models were well calibrated and their predictions correlated with the observed outcome at 3, 5, and 7 years in both development and validation sets. In comparison to existing prognostic models, the present models showed superior performance, as indicated by C-indices ranging from 0.722 to 0.755 (all P <0.0001) for PFS and from 0.680 to 0.744 (all P <0.0001) for OS. The predictive accuracy of the current models was robust in patients with clear-cell and non-clear-cell RCC.
Conclusions: Based on a prospective population-based patient cohort, the newly developed prognostic models were externally validated and outperformed the currently available models for predicting recurrence and survival in patients with non-metastatic RCC after surgery. The current models have the potential to aid in clinical trial design and facilitate clinical decision-making for both clear-cell and non-clear-cell RCC patients at varying risk of recurrence and survival.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
No authors reported disclosures.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Are Current Survival Prediction Tools Useful When Treating Subsequent Skeletal-related Events From Bone Metastases?Clin Orthop Relat Res. 2024 Sep 1;482(9):1710-1721. doi: 10.1097/CORR.0000000000003030. Epub 2024 Mar 22. Clin Orthop Relat Res. 2024. PMID: 38517402
-
[Clinicopathological features and survival analysis of TFE3-rearranged renal cell carcinoma with venous tumor thrombus].Beijing Da Xue Xue Bao Yi Xue Ban. 2025 Aug 18;57(4):650-661. doi: 10.19723/j.issn.1671-167X.2025.04.004. Beijing Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40754899 Free PMC article. Chinese.
-
The comparative and added prognostic value of biomarkers to the Revised Cardiac Risk Index for preoperative prediction of major adverse cardiac events and all-cause mortality in patients who undergo noncardiac surgery.Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD013139. doi: 10.1002/14651858.CD013139.pub2. Cochrane Database Syst Rev. 2021. PMID: 34931303 Free PMC article.
-
First-line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2023 May 4;5(5):CD013798. doi: 10.1002/14651858.CD013798.pub2. Cochrane Database Syst Rev. 2023. PMID: 37146227 Free PMC article.
Cited by
-
Hot Spots in Urogenital Basic Cancer Research and Clinics.Cancers (Basel). 2025 Mar 31;17(7):1173. doi: 10.3390/cancers17071173. Cancers (Basel). 2025. PMID: 40227699 Free PMC article. Review.
-
Prognostic Value of B7-H3 and a Novel Scoring System in Localized Renal Cell Carcinoma.Medicina (Kaunas). 2025 May 9;61(5):867. doi: 10.3390/medicina61050867. Medicina (Kaunas). 2025. PMID: 40428825 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. . European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol 2019;75:799–810. - PubMed
-
- Powles T, Albiges L, Bex A, et al. . ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol 2021;32:1511–1519. - PubMed
-
- Capitanio U, Montorsi F. Renal cancer. Lancet 2016;387:894–906. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

