Expanding the Coordination of f-Block Metals with Tris[2-(2-methoxyethoxy)ethyl]amine: From Molecular Complexes to Cage-like Structures
- PMID: 38016147
- PMCID: PMC11129929
- DOI: 10.1021/acs.inorgchem.3c02752
Expanding the Coordination of f-Block Metals with Tris[2-(2-methoxyethoxy)ethyl]amine: From Molecular Complexes to Cage-like Structures
Abstract
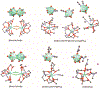
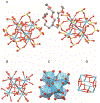
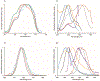
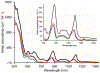
Low-valent f-block metals have intrinsic luminescence, electrochemical, and magnetic properties that are modulated with ligands, causing the coordination chemistry of these metals to be imperative to generating critical insights needed to impact modern applications. To this end, we synthesized and characterized a series of twenty-seven complexes of f-metal ions including EuII, YbII, SmII, and UIII and hexanuclear clusters of LaIII and CeIII to study the impact of tris[2-(2-methoxyethoxy)ethyl]amine, a flexible acyclic analogue of the extensively studied 2.2.2-cryptand, on the coordination chemistry and photophysical properties of low-valent f-block metals. We demonstrate that the flexibility of the ligand enables luminescence tunability over a greater range than analogous cryptates of EuII in solution. Furthermore, the ligand also displays a variety of binding modes to f-block metals in the solid state that are inaccessible to cryptates of low-valent f-block metals. In addition to serving as a ligand for f-block metals of various sizes and oxidation states, tris[2-(2-methoxyethoxy)ethyl]amine also deprotonates water molecules coordinated to trivalent triflate salts of f-block metal ions, enabling the isolation of hexanuclear clusters containing either LaIII or CeIII. The ligand was also found to bind more tightly to YbII and UIII in the solid state compared to 2.2.2-cryptand, suggesting that it can play a role in the isolation of other low-valent f-block metals such CfII, NpIII, and PuIII. We expect that our findings will inspire applications of tris[2-(2-methoxyethoxy)ethyl]amine in the design of light-emitting diodes and the synthesis of extremely reducing divalent f-block metal complexes that are of interest for a wide range of applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Dye JL Electrides: Early Examples of Quantum Confinement. Acc. Chem. Res 2009, 42, 1564–1572. - PubMed
-
- Dye JL Electrides: Ionic Salts with Electrons as the Anions. Science 1990, 247, 663–668. - PubMed
-
- Xie Q; Huang RH; Ichimura AS; Phillips RC; Pratt WP Jr.; Dye JL Structure and Properties of a New Electride, Rb+(cryptand[2.2.2])e−−. J. Am. Chem. Soc 2000, 122, 6971–6978. - PubMed
-
- Tehan FJ; Barnett BL; Dye JL Alkali Anions. Preparation and Crystal Structure of a Compound Which Contains the Cryptated Sodium Cation and the Sodium Anion. J. Am. Chem. Soc 1974, 96, 7203–7208.
-
- Lok MT; Tehan FJ; Dye JL Spectra of Na−−, K−−, and e−−solv in Amines and Ethers. J. Phys. Chem 1972, 76, 2975–2981.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

