Probing Multiple Transplant Delivery Routes of CD+34 Stem Cells for Promoting Behavioral and Histological Benefits in Experimental Ischemic Stroke
- PMID: 38016184
- PMCID: PMC10872715
- DOI: 10.1093/stcltm/szad081
Probing Multiple Transplant Delivery Routes of CD+34 Stem Cells for Promoting Behavioral and Histological Benefits in Experimental Ischemic Stroke
Abstract
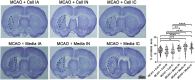
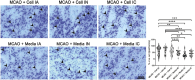
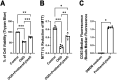
Stroke is a leading cause of death in the US and around the world but with limited treatment options. Survivors often present with long-term cognitive and neurological deficits. Stem cell-based therapy has emerged as a potential treatment for stroke. While stem cell transplantation in stroke has reached clinical trials, mostly safety outcomes have been reported with efficacy readouts warranting more studies. In an effort to optimize the stem cell regimen for stroke, here we conducted vis-a-vis comparison of different routes of transplantation, namely, intracerebral, intraarterial, and intranasal delivery of expanded human CD34 + stem cells, called ProtheraCytes, in the established stroke model of transient middle cerebral artery occlusion (MCAO) using adult Sprague-Dawley rats. After adjusting for the dose and subacute timing of cell delivery, animals were randomly assigned to receive either ProtheraCytes or vehicle. Motor and neurological assays from days 7 to 28 post-stroke revealed significant functional recovery across all 3 delivery routes of ProtheraCytes compared to vehicle-treated stroke rats. Additionally, ProtheraCytes-transplanted stroke rats displayed significantly reduced infarct size and cell loss in the peri-infarct area coupled with enhanced neurogenesis and angiogenesis compared to vehicle-treated stroke rats. These results highlight the safety and efficacy of transplanting ProtheraCytes, including via the minimally invasive intranasal route, in conferring robust and stable behavioral and histological positive outcomes in experimental stroke.
Keywords: angiogenesis; cell delivery route; cell transplantation; cerebral ischemia; functional recovery; neurogenesis.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
C.V., I.G. declared employment, patent holder and stock ownership with CellProthera. C.B. declared research funding from NIH. The other authors declared no potential conflicts of interest.
Figures







Similar articles
-
Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats.Brain Res. 2018 Feb 1;1680:143-154. doi: 10.1016/j.brainres.2017.12.017. Epub 2017 Dec 21. Brain Res. 2018. PMID: 29274877
-
Nose-to-brain delivery of stem cells in stroke: the role of extracellular vesicles.Stem Cells Transl Med. 2024 Nov 12;13(11):1043-1052. doi: 10.1093/stcltm/szae072. Stem Cells Transl Med. 2024. PMID: 39401332 Free PMC article. Review.
-
Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation.Stroke. 2013 Dec;44(12):3473-81. doi: 10.1161/STROKEAHA.113.001943. Epub 2013 Oct 15. Stroke. 2013. PMID: 24130140 Free PMC article.
-
The three-phase enriched environment paradigm promotes neurovascular restorative and prevents learning impairment after ischemic stroke in rats.Neurobiol Dis. 2020 Dec;146:105091. doi: 10.1016/j.nbd.2020.105091. Epub 2020 Sep 23. Neurobiol Dis. 2020. PMID: 32979506
-
Trophic factors and cell therapy to stimulate brain repair after ischaemic stroke.J Cell Mol Med. 2012 Oct;16(10):2280-90. doi: 10.1111/j.1582-4934.2012.01575.x. J Cell Mol Med. 2012. PMID: 22452968 Free PMC article. Review.
Cited by
-
Extracellular vesicle therapy in neurological disorders.J Biomed Sci. 2024 Aug 25;31(1):85. doi: 10.1186/s12929-024-01075-w. J Biomed Sci. 2024. PMID: 39183263 Free PMC article. Review.
-
Endogenous Extracellular Vesicles Participate in Brain Remodeling after Ischemic Stroke.Int J Mol Sci. 2023 Nov 28;24(23):16857. doi: 10.3390/ijms242316857. Int J Mol Sci. 2023. PMID: 38069179 Free PMC article.
-
Enriched environment inhibits GLT-1 ubiquitination by downregulating SMURF1 to attenuate ischemic brain injury induced excitotoxicity.Cell Biosci. 2025 Aug 28;15(1):124. doi: 10.1186/s13578-025-01457-z. Cell Biosci. 2025. PMID: 40877964 Free PMC article.
-
Intra-Arterial Administration of Stem Cells and Exosomes for Central Nervous System Disease.Int J Mol Sci. 2025 Jul 31;26(15):7405. doi: 10.3390/ijms26157405. Int J Mol Sci. 2025. PMID: 40806534 Free PMC article. Review.
-
Stem Cells Run Like Clockwork for Stroke Therapeutics.Stem Cell Rev Rep. 2025 Jul 18. doi: 10.1007/s12015-025-10895-8. Online ahead of print. Stem Cell Rev Rep. 2025. PMID: 40676475 No abstract available.
References
-
- Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. Epub 20131218. 10.1161/01.cir.0000441139.02102.80 - DOI - PMC - PubMed
-
- Ovbiagele B, Goldstein LB, Higashida RT, et al. ; American Heart Association Advocacy Coordinating Committee and Stroke Council. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44(8):2361-75. Epub 20130522. 10.1161/STR.0b013e31829734f2 - DOI - PubMed
-
- Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-35. Epub 20140805. 10.1016/S0140-6736(14)60584-5 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

