Modern Photodynamic Glioblastoma Therapy Using Curcumin- or Parietin-Loaded Lipid Nanoparticles in a CAM Model Study
- PMID: 38016693
- PMCID: PMC10732153
- DOI: 10.1021/acsabm.3c00695
Modern Photodynamic Glioblastoma Therapy Using Curcumin- or Parietin-Loaded Lipid Nanoparticles in a CAM Model Study
Abstract
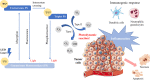
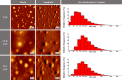
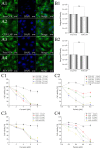
Natural photosensitizers, such as curcumin or parietin, play a vital role in photodynamic therapy (PDT), causing a light-mediated reaction that kills cancer cells. PDT is a promising treatment option for glioblastoma, especially when combined with nanoscale drug delivery systems. The curcumin- or parietin-loaded lipid nanoparticles were prepared via dual asymmetric centrifugation and subsequently characterized through physicochemical analyses including dynamic light scattering, laser Doppler velocimetry, and atomic force microscopy. The combination of PDT and lipid nanoparticles has been evaluated in vitro regarding uptake, safety, and efficacy. The extensive and well-vascularized chorioallantois membrane (CAM) of fertilized hen's eggs offers an optimal platform for three-dimensional cell culture, which has been used in this study to evaluate the photodynamic efficacy of lipid nanoparticles against glioblastoma cells. In contrast to other animal models, the CAM model lacks a mature immune system in an early stage, facilitating the growth of xenografts without rejection. Treatment of xenografted U87 glioblastoma cells on CAM was performed to assess the effects on tumor viability, growth, and angiogenesis. The xenografts and the surrounding blood vessels were targeted through topical application, and the effects of photodynamic therapy have been confirmed microscopically and via positron emission tomography and X-ray computed tomography. Finally, the excised xenografts embedded in the CAM were analyzed histologically by hematoxylin and eosin and KI67 staining.
Keywords: 3D cell culture; CAM model; drug delivery system; dual asymmetric centrifugation; lipid nanoparticles; natural photosensitizer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Buckner J. C.; Shaw E. G.; Pugh S. L.; Chakravarti A.; Gilbert M. R.; Barger G. R.; Coons S.; Ricci P.; Bullard D.; Brown P. D.; Stelzer K.; Brachman D.; Suh J. H.; Schultz C. J.; Bahary J.-P.; Fisher B. J.; Kim H.; Murtha A. D.; Bell E. H.; Won M.; Mehta M. P.; Curran W. J. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. New England journal of medicine 2016, 374 (14), 1344–1355. 10.1056/NEJMoa1500925. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

