Randomized, open-label, phase II, biomarker study of immune-mediated mechanism of action of neoadjuvant subcutaneous trastuzumab in patients with locally advanced, inflammatory, or early HER2-positive breast cancer-Immun-HER trial (GOIRC-01-2016)
- PMID: 38016718
- PMCID: PMC10685938
- DOI: 10.1136/jitc-2023-007667
Randomized, open-label, phase II, biomarker study of immune-mediated mechanism of action of neoadjuvant subcutaneous trastuzumab in patients with locally advanced, inflammatory, or early HER2-positive breast cancer-Immun-HER trial (GOIRC-01-2016)
Abstract
Background: It is possible to induce immunomodulation in HER2-positive breast cancer (BC) by modifying the route of administration of trastuzumab.
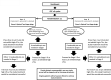
Methods: In this multicenter randomized phase II trial, all enrolled patients (pts) with T2-T4d HER2-positive BC received 3 cycles of neoadjuvant treatment (NAT) with fluorouracil, epirubicin and cyclophosphamide every 3 weeks (q21), followed by docetaxel/pertuzumab plus intravenous trastuzumab (arm A) or, docetaxel/pertuzumab plus subcutaneous (SC) trastuzumab (arm B) q21x4 cycles. After surgical operation, each pt was treated with trastuzumab q21x14 cycles using the same SC or intravenous formulation of NAT. Primary endpoint was the proportion of subjects with high stromal tumor-infiltrating lymphocytes (sTILs) in postneoadjuvant residual disease (RD).
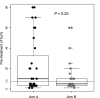
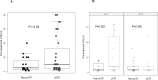
Results: Sixty-three pts (31 (arm A) and 32 (arm B)) were enrolled. Pathological complete response was obtained by 20/31 pts (64.5%; 95% CI 45.4% to 80.1%) in arm A and 19/32 pts (59.4%; 95% CI 40.1% to 76.3%) in arm B. High sTILs were observed in 27% and 46% of postneoadjuvant residual tumors in arms A and B, respectively. CD8+ T cells increased significantly in RDs of both arms (p=0.014 and 0.002 for arm A and B, respectively), whereas a significant decline in the level of CD4+ FoxP3+ regulatory T cells was observed only in arm B (p=0.016). A significant upregulation of PD-1 on sTILs was found in RD of pts enrolled in arm B (p=0.012), while programmed death-ligand 1 (PD-L1) was significantly overexpressed in residual tumors of arm A (p=0.02). A strong negative correlation was reported in arm B between expression of PD-L1 on pretreatment sTILs and CD3 expression on sTILs in RD (τ: -0.73). Grade≥3 AE incidence rates were similar between the two arms.
Conclusions: SC trastuzumab induced relevant sTILs enrichment, with favorable variations of immune parameters in HER2-positive BC pts with RD after NAT. Novel immunotherapy strategies should be tested to achieve SC-specific, antitumor immune response.
Trial registration number: NCT03144947, and EudraCT number: 2016-000435-41.
Keywords: adaptive Immunity; antibodies, neoplasm; breast neoplasms.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BP reports research grants from Lilly, Pfizer, Novartis; and personal fees from MSD outside the submitted work. EC reports travel grants from Pfizer and Ipsen outside the submitted work. EB reports research grants from Roche, AstraZeneca; personal fees from MSD, AstraZeneca, BMS, Roche, Novartis, Pfizer, Lilly; and travel grants from Roche and AstraZeneca outside the submitted work. LC reports personal fees from AstraZeneca, Novartis and Lilly outside the submitted work. LG reports personal fees from Daiichi, Seagen; and travel grants from Ipsen, Novartis, Pfizer outside the submitted work. AZ reports personal fees from AstraZeneca, Lilly, Pfizer, Daiichi, MSD, Roche, Seagen, Exact Sciences, Gilead and Istituto Gentili outside the submitted work. FM reports personal fees from Roche, Novartis, AstraZeneca, Daiichi, Seagen, MSD, Lilly, Pfizer, Pierre Fabre; and travel grants from Roche and AstraZeneca outside the submitted work; from May 15th 2023, FM is Roche employee. AM reports research grants from Lilly; personal fees from Seagen, Daiichi, Gilead, Novartis, AstraZeneca; and travel grants from Gilead and Novartis outside the submitted work.
Figures


References
-
- Gianni L, Pienkowski T, Im Y-H, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (Neosphere): a randomised Multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. 10.1016/S1470-2045(11)70336-9 - DOI - PubMed
-
- Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and Trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2+ early breast cancer. Cancer Res 2016;76:S5–04. 10.1158/1538-7445.SABCS15-S5-04 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
