Schisandrin B, a dual positive allosteric modulator of GABAA and glycine receptors, alleviates seizures in multiple mouse models
- PMID: 38017298
- PMCID: PMC10834591
- DOI: 10.1038/s41401-023-01195-3
Schisandrin B, a dual positive allosteric modulator of GABAA and glycine receptors, alleviates seizures in multiple mouse models
Abstract
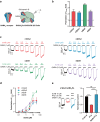
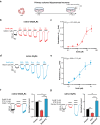
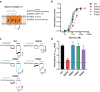
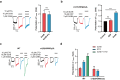
Epilepsy is a prevalent and severe neurological disorder and approximately 30% of patients are resistant to existing medications. It is of utmost importance to develop alternative therapies to treat epilepsy. Schisandrin B (SchB) is a major bioactive constituent of Schisandra chinensis (Turcz.) Baill and has multiple neuroprotective effects, sedative and hypnotic activities. In this study, we investigated the antiseizure effect of SchB in various mouse models of seizure and explored the underlying mechanisms. Pentylenetetrazole (PTZ), strychnine (STR), and pilocarpine-induced mouse seizure models were established. We showed that injection of SchB (10, 30, 60 mg/kg, i.p.) dose-dependently delayed the onset of generalized tonic-clonic seizures (GTCS), reduced the incidence of GTCS and mortality in PTZ and STR models. Meanwhile, injection of SchB (30 mg/kg, i.p.) exhibited therapeutic potential in pilocarpine-induced status epilepticus model, which was considered as a drug-resistant model. In whole-cell recording from CHO/HEK-239 cells stably expressing recombinant human GABAA receptors (GABAARs) and glycine receptors (GlyRs) and cultured hippocampal neurons, co-application of SchB dose-dependently enhanced GABA or glycine-induced current with EC50 values at around 5 μM, and application of SchB (10 μM) alone did not activate the channels in the absence of GABA or glycine. Furthermore, SchB (10 μM) eliminated both PTZ-induced inhibition on GABA-induced current (IGABA) and strychnine (STR)-induced inhibition on glycine-induced current (Iglycine). Moreover, SchB (10 μM) efficiently rescued the impaired GABAARs associated with genetic epilepsies. In addition, the homologous mutants in both GlyRs-α1(S267Q) and GABAARs-α1(S297Q)β2(N289S)γ2L receptors by site-directed mutagenesis tests abolished SchB-induced potentiation of IGABA and Iglycine. In conclusion, we have identified SchB as a natural positive allosteric modulator of GABAARs and GlyRs, supporting its potential as alternative therapies for epilepsy.
Keywords: GABAA receptors; Schisandrin B; epilepsy; glycine receptors.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

