Disrupted hypothalamic transcriptomics and proteomics in a mouse model of type 2 diabetes exposed to recurrent hypoglycaemia
- PMID: 38017352
- PMCID: PMC10789691
- DOI: 10.1007/s00125-023-06043-x
Disrupted hypothalamic transcriptomics and proteomics in a mouse model of type 2 diabetes exposed to recurrent hypoglycaemia
Erratum in
-
Correction to: Disrupted hypothalamic transcriptomics and proteomics in a mouse model of type 2 diabetes exposed to recurrent hypoglycaemia.Diabetologia. 2024 Feb;67(2):403. doi: 10.1007/s00125-023-06083-3. Diabetologia. 2024. PMID: 38206364 Free PMC article. No abstract available.
Abstract
Aims/hypothesis: Repeated exposures to insulin-induced hypoglycaemia in people with diabetes progressively impairs the counterregulatory response (CRR) that restores normoglycaemia. This defect is characterised by reduced secretion of glucagon and other counterregulatory hormones. Evidence indicates that glucose-responsive neurons located in the hypothalamus orchestrate the CRR. Here, we aimed to identify the changes in hypothalamic gene and protein expression that underlie impaired CRR in a mouse model of defective CRR.
Methods: High-fat-diet fed and low-dose streptozocin-treated C57BL/6N mice were exposed to one (acute hypoglycaemia [AH]) or multiple (recurrent hypoglycaemia [RH]) insulin-induced hypoglycaemic episodes and plasma glucagon levels were measured. Single-nuclei RNA-seq (snRNA-seq) data were obtained from the hypothalamus and cortex of mice exposed to AH and RH. Proteomic data were obtained from hypothalamic synaptosomal fractions.
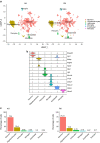
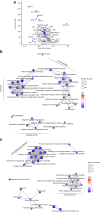
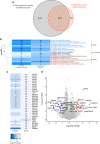
Results: The final insulin injection resulted in similar plasma glucose levels in the RH group and AH groups, but glucagon secretion was significantly lower in the RH group (AH: 94.5±9.2 ng/l [n=33]; RH: 59.0±4.8 ng/l [n=37]; p<0.001). Analysis of snRNA-seq data revealed similar proportions of hypothalamic cell subpopulations in the AH- and RH-exposed mice. Changes in transcriptional profiles were found in all cell types analysed. In neurons from RH-exposed mice, we observed a significant decrease in expression of Avp, Pmch and Pcsk1n, and the most overexpressed gene was Kcnq1ot1, as compared with AH-exposed mice. Gene ontology analysis of differentially expressed genes (DEGs) indicated a coordinated decrease in many oxidative phosphorylation genes and reduced expression of vacuolar H+- and Na+/K+-ATPases; these observations were in large part confirmed in the proteomic analysis of synaptosomal fractions. Compared with AH-exposed mice, oligodendrocytes from RH-exposed mice had major changes in gene expression that suggested reduced myelin formation. In astrocytes from RH-exposed mice, DEGs indicated reduced capacity for neurotransmitters scavenging in tripartite synapses as compared with astrocytes from AH-exposed mice. In addition, in neurons and astrocytes, multiple changes in gene expression suggested increased amyloid beta (Aβ) production and stability. The snRNA-seq analysis of the cortex showed that the adaptation to RH involved different biological processes from those seen in the hypothalamus.
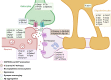
Conclusions/interpretation: The present study provides a model of defective counterregulation in a mouse model of type 2 diabetes. It shows that repeated hypoglycaemic episodes induce multiple defects affecting all hypothalamic cell types and their interactions, indicative of impaired neuronal network signalling and dysegulated hypoglycaemia sensing, and displaying features of neurodegenerative diseases. It also shows that repeated hypoglycaemia leads to specific molecular adaptation in the hypothalamus when compared with the cortex.
Data availability: The transcriptomic dataset is available via the GEO ( http://www.ncbi.nlm.nih.gov/geo/ ), using the accession no. GSE226277. The proteomic dataset is available via the ProteomeXchange data repository ( http://www.proteomexchange.org ), using the accession no. PXD040183.
Keywords: Astrocytes; Counterregulation; Glucagon; Hypoglycaemia; Hypothalamus; Insulin; Neurodegeneration; Neurons; Oligodendrocytes; RNA-seq.
© 2023. The Author(s).
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

