Long noncoding RNA KCNMA1-AS1 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by activating the SMAD9 signaling pathway
- PMID: 38017487
- PMCID: PMC10685465
- DOI: 10.1186/s13062-023-00425-2
Long noncoding RNA KCNMA1-AS1 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by activating the SMAD9 signaling pathway
Abstract
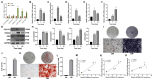
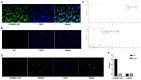
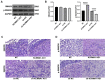
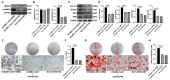
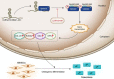
The human bone marrow mesenchymal stem cells (hBMSCs) undergo intense osteogenic differentiation, a crucial bone formation mechanism. Evidence from prior studies suggested an association between long noncoding RNAs (lncRNAs) and the osteogenic differentiation of hBMSCs. However, precise roles and molecular mechanisms are still largely unknown. In this work, we report for the first time that lncRNA KCNMA1 antisense RNA 1 (KCNMA1-AS1) plays a vital role in regulating hBMSCs' osteogenic differentiation. Here, it was observed that the KCNMA1-AS1 expression levels were significantly upregulated during osteogenic differentiation. In addition, KCNMA1-AS1 overexpression enhanced in vitro osteogenic differentiation of hBMSCs and in vivo bone formation, whereas knockdown of KCNMA1-AS1 resulted in the opposite result. Additionally, the interaction between KCNMA1-AS1 and mothers against decapentaplegic homolog 9 (SMAD9) was confirmed by an RNA pull-down experiment, mass spectrometry, and RIP assay. This interaction regulated the activation of the SMAD9 signaling pathway. Moreover, rescue assays demonstrated that the inhibitor of the SMAD9 signaling pathway reversed the stimulative effects on osteogenic differentiation of hBMSCs by KCNMA1-AS1 overexpression. Altogether, our results stipulate that KCNMA1-AS1 promotes osteogenic differentiation of hBMSCs via activating the SMAD9 signaling pathway and can serve as a biomarker and therapeutic target in treating bone defects.
Keywords: Human bone marrow mesenchymal stem cells; KCNMA1-AS1; Osteogenic differentiation; SMAD9; lncRNA.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Tissue-engineered autologous. grafts for facial bone reconstruction | Science Translational Medicine [Internet]. [cited 2023 Sep 14]. Available from: https://www.science.org/doi/abs/10.1126/scitranslmed.aad5904. - DOI - PMC - PubMed
-
- Cells | Free Full-Text. | Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy [Internet]. [cited 2023 Sep 14]. Available from: https://www.mdpi.com/2073-4409/10/11/2993. - PMC - PubMed
-
- Park JJ, Rochlin DH, Parsaei Y, Shetye PR, Witek L, Leucht P et al. Bone tissue Engineering Strategies for Alveolar Cleft: review of preclinical results and guidelines for Future studies. Cleft Palate Craniofac J. 2022;10556656221104954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

