Effects of Lianhuaqingwen Capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial
- PMID: 38017515
- PMCID: PMC10685492
- DOI: 10.1186/s12985-023-02144-6
Effects of Lianhuaqingwen Capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial
Abstract
Background: In a randomized trial, Lianhuaqingwen (LHQW) capsule was effective for accelerating symptom recovery among patients with coronavirus disease 2019 (COVID-19). However, the lack of blinding and limited sample sizes decreased the level of clinical evidence.
Objectives: To evaluate the efficacy and safety of LHQW capsule in adults with mild-to-moderate COVID-19.
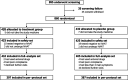
Methods: We conducted a double-blind randomized controlled trial in adults with mild-to-moderate COVID-19 (17 sites from China, Thailand, Philippine and Vietnam). Patients received standard-of-care alone or plus LHQW capsules (4 capsules, thrice daily) for 14 days. The primary endpoint was the median time to sustained clinical improvement or resolution of nine major symptoms.
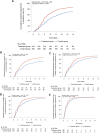
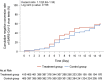
Results: The full-analysis set consisted of 410 patients in LHQW capsules and 405 in placebo group. LHQW significantly shortened the primary endpoint in the full-analysis set (4.0 vs. 6.7 days, hazards ratio: 1.63, 95% confidence interval: 1.39-1.90). LHQW capsules shortened the median time to sustained clinical improvement or resolution of stuffy or runny nose (2.8 vs. 3.7 days), sore throat (2.0 vs. 2.6 days), cough (3.2 vs. 4.9 days), feeling hot or feverish (1.0 vs. 1.3 days), low energy or tiredness (1.3 vs. 1.9 days), and myalgia (1.5 vs. 2.0 days). The duration to sustained clinical improvement or resolution of shortness of breath, headache, and chills or shivering did not differ significantly between the two groups. Safety was comparable between the two groups. No serious adverse events were reported.
Interpretation: LHQW capsules promote recovery of mild-to-moderate COVID-19 via accelerating symptom resolution and were well tolerated. Trial registration ChiCTR2200056727 .
Keywords: Coronavirus disease 2019; Inflammation; Lianhuaqingwen Capsule; Omicron; Symptom resolution.
© 2023. The Author(s).
Conflict of interest statement
None.
Figures


References
-
- World Health Organization Coronavirus (COVID-19) Dashboard [https://covid19.who.int/]
-
- Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, Hinsley W, Bernal JL, Kall M, Bhatt S, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. - DOI - PMC - PubMed
-
- Chen Z, Deng X, Fang L, Sun K, Wu Y, Che T, Zou J, Cai J, Liu H, Wang Y, et al. Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: a descriptive study. Lancet Reg Health West Pac. 2022;29:100592. doi: 10.1016/j.lanwpc.2022.100592. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

