Breaking barriers: exploring mechanisms behind opening the blood-brain barrier
- PMID: 38017530
- PMCID: PMC10683235
- DOI: 10.1186/s12987-023-00489-2
Breaking barriers: exploring mechanisms behind opening the blood-brain barrier
Abstract
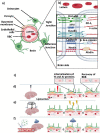
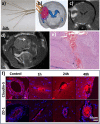
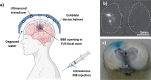
The blood-brain barrier (BBB) is a selectively permeable membrane that separates the bloodstream from the brain. While useful for protecting neural tissue from harmful substances, brain-related diseases are difficult to treat due to this barrier, as it also limits the efficacy of drug delivery. To address this, promising new approaches for enhancing drug delivery are based on disrupting the BBB using physical means, including optical/photothermal therapy, electrical stimulation, and acoustic/mechanical stimulation. These physical mechanisms can temporarily and locally open the BBB, allowing drugs and other substances to enter. Focused ultrasound is particularly promising, with the ability to focus energies to targeted, deep-brain regions. In this review, we examine recent advances in physical approaches for temporary BBB disruption, describing their underlying mechanisms as well as evaluating the utility of these physical approaches with regard to their potential risks and limitations. While these methods have demonstrated efficacy in disrupting the BBB, their safety, comparative efficacy, and practicality for clinical use remain an ongoing topic of research.
Keywords: BBB stimulation; Blood–Brain barrier; Focused ultrasound; Neurological therapeutics; Tight junctions.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

