Microglial function, INPP5D/SHIP1 signaling, and NLRP3 inflammasome activation: implications for Alzheimer's disease
- PMID: 38017562
- PMCID: PMC10685641
- DOI: 10.1186/s13024-023-00674-9
Microglial function, INPP5D/SHIP1 signaling, and NLRP3 inflammasome activation: implications for Alzheimer's disease
Abstract
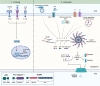
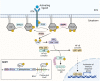
Recent genetic studies on Alzheimer's disease (AD) have brought microglia under the spotlight, as loci associated with AD risk are enriched in genes expressed in microglia. Several of these genes have been recognized for their central roles in microglial functions. Increasing evidence suggests that SHIP1, the protein encoded by the AD-associated gene INPP5D, is an important regulator of microglial phagocytosis and immune response. A recent study from our group identified SHIP1 as a negative regulator of the NLRP3 inflammasome in human iPSC-derived microglial cells (iMGs). In addition, we found evidence for a connection between SHIP1 activity and inflammasome activation in the AD brain. The NLRP3 inflammasome is a multiprotein complex that induces the secretion of pro-inflammatory cytokines as part of innate immune responses against pathogens and endogenous damage signals. Previously published studies have suggested that the NLRP3 inflammasome is activated in AD and contributes to AD-related pathology. Here, we provide an overview of the current understanding of the microglial NLRP3 inflammasome in the context of AD-related inflammation. We then review the known intracellular functions of SHIP1, including its role in phosphoinositide signaling, interactions with microglial phagocytic receptors such as TREM2 and evidence for its intersection with NLRP3 inflammasome signaling. Through rigorous examination of the intricate connections between microglial signaling pathways across several experimental systems and postmortem analyses, the field will be better equipped to tailor newly emerging therapeutic strategies targeting microglia in neurodegenerative diseases.
Keywords: Alzheimer’s Disease; INPP5D; Inflammation; Microglia; NLRP3 inflammasome; Phosphoinositide signaling; SHIP1.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

