Unveiling Candida albicans intestinal carriage in healthy volunteers: the role of micro- and mycobiota, diet, host genetics and immune response
- PMID: 38017705
- PMCID: PMC10732203
- DOI: 10.1080/19490976.2023.2287618
Unveiling Candida albicans intestinal carriage in healthy volunteers: the role of micro- and mycobiota, diet, host genetics and immune response
Abstract
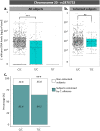
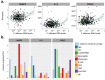
Candida albicans is a commensal yeast present in the gut of most healthy individuals but with highly variable concentrations. However, little is known about the host factors that influence colonization densities. We investigated how microbiota, host lifestyle factors, and genetics could shape C. albicans intestinal carriage in 695 healthy individuals from the Milieu Intérieur cohort. C. albicans intestinal carriage was detected in 82.9% of the subjects using quantitative PCR. Using linear mixed models and multiway-ANOVA, we explored C. albicans intestinal levels with regard to gut microbiota composition and lifestyle factors including diet. By analyzing shotgun metagenomics data and C. albicans qPCR data, we showed that Intestinimonas butyriciproducens was the only gut microbiota species whose relative abundance was negatively correlated with C. albicans concentration. Diet is also linked to C. albicans growth, with eating between meals and a low-sodium diet being associated with higher C. albicans levels. Furthermore, by Genome-Wide Association Study, we identified 26 single nucleotide polymorphisms suggestively associated with C. albicans colonization. In addition, we found that the intestinal levels of C. albicans might influence the host immune response, specifically in response to fungal challenge. We analyzed the transcriptional levels of 546 immune genes and the concentration of 13 cytokines after whole blood stimulation with C. albicans cells and showed positive associations between the extent of C. albicans intestinal levels and NLRP3 expression, as well as secreted IL-2 and CXCL5 concentrations. Taken together, these findings open the way for potential new interventional strategies to curb C. albicans intestinal overgrowth.
Keywords: Candida albicans; GWAS; colonization resistance; host factors; lifestyle factors; metagenomics; microbiota; mycobiota.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Zhai B, Ola M, Rolling T, Tosini NL, Joshowitz S, Littmann ER, Amoretti LA, Fontana E, Wright RJ, Miranda E, et al. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat Med. 2020;26(1):59–64. doi:10.1038/s41591-019-0709-7. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
