Eravacycline, the first four years: health outcomes and tolerability data for 19 hospitals in 5 U.S. regions from 2018 to 2022
- PMID: 38018984
- PMCID: PMC10782980
- DOI: 10.1128/spectrum.02351-23
Eravacycline, the first four years: health outcomes and tolerability data for 19 hospitals in 5 U.S. regions from 2018 to 2022
Abstract
The rise of multidrug-resistant (MDR) pathogens, especially MDR Gram-negatives, poses a significant challenge to clinicians and public health. These resilient bacteria have rendered many traditional antibiotics ineffective, underscoring the urgency for innovative therapeutic solutions. Eravacycline, a broad-spectrum fluorocycline tetracycline antibiotic approved by the FDA in 2018, emerges as a promising candidate, exhibiting potential against a diverse array of MDR bacteria, including Gram-negative, Gram-positive, anaerobic strains, and Mycobacterium. However, comprehensive data on its real-world application remain scarce. This retrospective cohort study, one of the largest of its kind, delves into the utilization of eravacycline across various infectious conditions in the USA during its initial 4 years post-FDA approval. Through assessing clinical, microbiological, and tolerability outcomes, the research offers pivotal insights into eravacycline's efficacy in addressing the pressing global challenge of MDR bacterial infections.
Keywords: antimicrobial stewardship; eravacycline; multidrug-resistant.
Conflict of interest statement
S.A. is a current employee of Nestle Health Sciences. T.M. is currently funded through Stellus Rx and has participated in scientific advisory boards for AbbVie Inc. and Basilea Pharmaceutica. J.A. is a speaker for Shionogi Inc. M.A.K. is a speaker for Tetraphase. M.P.V. received research funding from Paratek Pharmaceuticals, Cumberland Pharmaceuticals, NIAID, and advisory Boards for Ferring Pharmaceuticals, Melinta Therapeutics, and Merck & Co. K.C.M. has consulted for Shionogi. B.M.J. has participated in speaking bureaus for Abbvie, La Jolla, and Paratek. A.L.V.H. has participated in speaking bureaus and has received research funding from Tetraphase. M.J.R. has received research and consulting from or participated in speaking bureaus for Abbvie, Melinta, Merck, Paratek, Shionogi, T2 Biosystems, and Tetraphase (La Jolla). All other authors declare no conflict of interest.
Figures
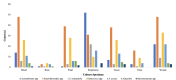

References
-
- Tacconelli E. 2017. World health organization. global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics 2017. Available from: https://www.who.int/medicines/publications/WHO-PPLShort_Summary_25Feb-ET...
-
- CDC . 2021. Antibiotic-resistant germs: new threats. Available from: https://www.cdc.gov/drugresistance/biggest-threats.html
-
- XERAVA (eravacycline) for injection. 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

