Comprehensive, comparative evaluation of 25 automated SARS-CoV-2 serology assays
- PMID: 38018986
- PMCID: PMC10783060
- DOI: 10.1128/spectrum.03228-23
Comprehensive, comparative evaluation of 25 automated SARS-CoV-2 serology assays
Abstract
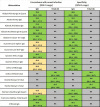
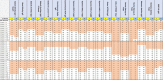
We have previously highlighted the fact that hundreds of SARS-CoV-2 serology tests were released months after the onset of the COVID-19 pandemic. Of the hundreds of studies investigating the test kits' performance, few were comparative reports, using the same comprehensive sample set across multiple tests. Recently, we reported a comparative assessment of 35 rapid diagnostic tests (RDTs) or microtiter plate enzyme immunoassays (EIA) for use in low- and middle-income countries, using a large sample set from individuals with a history of COVID-19. Only a few tests meet WHO Target Product Profile performance requirements. This study reports on the performance of a further 25 automated SARS-CoV-2 immunoassays using the same panel of samples. The results highlight the better analytical and clinical performance of automated serology test kits compared with RDTs, and the importance of independent comparative assessments to inform the use and procurement of these tests for both diagnostic and epidemiological investigations.
Keywords: anti-SARS-CoV-2 antibodies; automated assays; evaluation; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Comprehensive, Comparative Evaluation of 35 Manual SARS-CoV-2 Serological Assays.Microbiol Spectr. 2023 Jun 15;11(3):e0510122. doi: 10.1128/spectrum.05101-22. Epub 2023 May 9. Microbiol Spectr. 2023. PMID: 37158743 Free PMC article.
-
Clinical diagnostic performance evaluation of five immunoassays for antibodies to SARS-CoV-2 diagnosis in a real-life routine care setting.Pan Afr Med J. 2021 May 3;39:3. doi: 10.11604/pamj.2021.39.3.26471. eCollection 2021. Pan Afr Med J. 2021. PMID: 34178231 Free PMC article.
-
Clinical evaluation of five different automated SARS-CoV-2 serology assays in a cohort of hospitalized COVID-19 patients.J Clin Virol. 2020 Sep;130:104549. doi: 10.1016/j.jcv.2020.104549. Epub 2020 Jul 17. J Clin Virol. 2020. PMID: 32763809 Free PMC article.
-
Diagnostic performance of COVID-19 serology assays.Malays J Pathol. 2020 Apr;42(1):13-21. Malays J Pathol. 2020. PMID: 32342927 Review.
-
Review of Clinical Performance of Serology Based Commercial Diagnostic Assays for Detection of Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies.Viral Immunol. 2022 Mar;35(2):82-111. doi: 10.1089/vim.2020.0313. Epub 2022 Jan 10. Viral Immunol. 2022. PMID: 35007431 Review.
Cited by
-
Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies.Int J Mol Sci. 2024 Jul 26;25(15):8155. doi: 10.3390/ijms25158155. Int J Mol Sci. 2024. PMID: 39125722 Free PMC article. Review.
References
-
- Peaper DR, Rhoads DD, Sullivan KV, Couturier MR, Humphries RM, Martin IW, Nolte FS, Rowlinson MC, She RC, Simner PJ, Theel ES, Wojewoda CM. 2021. Considerations from the college of American pathologists for implementation of an assay for SARS-CoV-2 testing after a change in regulatory status. J Clin Microbiol 59:e0116721. doi:10.1128/JCM.01167-21 - DOI - PMC - PubMed
-
- Black MA, Shen G, Feng X, Garcia Beltran WF, Feng Y, Vasudevaraja V, Allison D, Lin LH, Gindin T, Astudillo M, Yang D, Murali M, Iafrate AJ, Jour G, Cotzia P, Snuderl M. 2021. Analytical performance of lateral flow immunoassay for SARS-CoV-2 exposure screening on venous and capillary blood samples. J Immunol Methods 489:112909. doi:10.1016/j.jim.2020.112909 - DOI - PMC - PubMed
-
- Chan A, Martinez-Cajas J, Yip PM, Kulasingam V, Garland J, Holland D, Shamseddin MK, Gong Y. 2023. Evaluation and comparison of four quantitative SARS-CoV-2 serological assays in COVID-19 patients and immunized healthy individuals, cancer patients, and patients with immunosuppressive therapy. Clin Biochem 116:1–6. doi:10.1016/j.clinbiochem.2023.02.010 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

