Integrated multi-omics reveals the roles of cecal microbiota and its derived bacterial consortium in promoting chicken growth
- PMID: 38018992
- PMCID: PMC10734529
- DOI: 10.1128/msystems.00844-23
Integrated multi-omics reveals the roles of cecal microbiota and its derived bacterial consortium in promoting chicken growth
Abstract
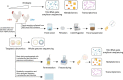
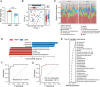
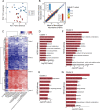
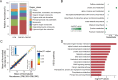
The improvement of chicken growth performance is one of the major concerns for the poultry industry. Gut microbes are increasingly evidenced to be associated with chicken physiology and metabolism, thereby influencing chicken growth and development. Here, through integrated multi-omics analyses, we showed that chickens from the same line differing in their body weight were very different in their gut microbiota structure and host-microbiota crosstalk; microbes in high body weight (HBW) chickens contributed to chicken growth by regulating the gut function and homeostasis. We also verified that a specific bacterial consortium consisting of isolates from the HBW chickens has the potential to be used as chicken growth promoters. These findings provide new insights into the potential links between gut microbiota and chicken phenotypes, shedding light on future manipulation of chicken gut microbiota to improve chicken growth performance.
Keywords: cecal microbiota; chicken; growth performance; metabolomics; targeted culturomics; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Comprehensive cultivation of the broiler gut microbiota guides bacterial isolation from chickens.Sci China Life Sci. 2025 Mar;68(3):836-845. doi: 10.1007/s11427-024-2735-8. Epub 2024 Nov 26. Sci China Life Sci. 2025. PMID: 39607604
-
Unraveling the gut microbiota of Tibetan chickens: insights into highland adaptation and ecological advantages.Microbiol Spectr. 2024 Nov 5;12(11):e0051924. doi: 10.1128/spectrum.00519-24. Epub 2024 Sep 30. Microbiol Spectr. 2024. PMID: 39345125 Free PMC article.
-
Cecal microbiome divergence of broiler chickens by sex and body weight.J Microbiol. 2017 Dec;55(12):939-945. doi: 10.1007/s12275-017-7202-0. Epub 2017 Dec 7. J Microbiol. 2017. PMID: 29214491
-
Cecal Microbial Succession and Its Apparent Association with Nutrient Metabolism in Broiler Chickens.mSphere. 2023 Jun 22;8(3):e0061422. doi: 10.1128/msphere.00614-22. Epub 2023 Apr 5. mSphere. 2023. PMID: 37017520 Free PMC article.
-
The Influence of Cecal Microbiota Transplantation on Chicken Injurious Behavior: Perspective in Human Neuropsychiatric Research.Biomolecules. 2024 Aug 16;14(8):1017. doi: 10.3390/biom14081017. Biomolecules. 2024. PMID: 39199404 Free PMC article. Review.
Cited by
-
Early fecal microbiota transplantation continuously improves chicken growth performance by inhibiting age-related Lactobacillus decline in jejunum.Microbiome. 2025 Feb 10;13(1):49. doi: 10.1186/s40168-024-02021-6. Microbiome. 2025. PMID: 39930537 Free PMC article.
-
Non-Targeted Metabolomics of Serum Reveals Biomarkers Associated with Body Weight in Wumeng Black-Bone Chickens.Animals (Basel). 2024 Sep 23;14(18):2743. doi: 10.3390/ani14182743. Animals (Basel). 2024. PMID: 39335332 Free PMC article.
-
Exploring the metabolic patterns and response mechanisms of bile acids during fasting: A study with poultry as an example.Poult Sci. 2025 Feb;104(2):104746. doi: 10.1016/j.psj.2024.104746. Epub 2024 Dec 31. Poult Sci. 2025. PMID: 39799857 Free PMC article.
-
Effects of a new compound probiotic on growth performance, antioxidant capacity, intestinal health, gut microbiota and metabolites of broilers.Poult Sci. 2025 Aug;104(8):105215. doi: 10.1016/j.psj.2025.105215. Epub 2025 Apr 25. Poult Sci. 2025. PMID: 40403549 Free PMC article.
-
Postbiotics: An Alternative for Improving Health and Performance of Poultry Production.Microorganisms. 2025 Jun 25;13(7):1472. doi: 10.3390/microorganisms13071472. Microorganisms. 2025. PMID: 40731983 Free PMC article. Review.
References
MeSH terms
Grants and funding
- 2022YFA1304201, 2021YFD1800400/MOST | National Key Research and Development Program of China (NKPs)
- 2021JJLH0084/Hainan Provincial Natural Science Foundation of China
- 6222032/| Natural Science Foundation of Beijing Municipality (Beijing Natural Science Foundation)
- Starting Grants Program for Young Talents at China Agricultural University
- 2115 Talent Development Program of China Agricultural University
LinkOut - more resources
Full Text Sources

