Gastric Acid Suppression Therapy and Its Association with Peritoneal Dialysis-Associated Peritonitis in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS)
- PMID: 38019215
- PMCID: PMC11000729
- DOI: 10.34067/KID.0000000000000325
Gastric Acid Suppression Therapy and Its Association with Peritoneal Dialysis-Associated Peritonitis in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS)
Abstract
Key Points:
In a large multinational cohort of PD patients, any GAS use was not associated with an increased risk of all-organism peritonitis.
For peritonitis, risks were particularly high among certain classes of organisms particularly for Gram-negative, enteric, and streptococcal peritonitis episodes.
The association with enteric peritonitis appeared to be stronger among H2RA users.
Background: Peritonitis is a major peritoneal dialysis–related complication. We determined whether gastric acid suppression (GAS) (proton pump inhibitor [PPI] or histamine-2 receptor antagonists [H2RAs]) use was associated with all-cause and organism-specific peritonitis in peritoneal dialysis patients.
Methods: In the Peritoneal Dialysis Outcomes and Practice Patterns Study (595 facilities, eight countries, years 2014–2022), associations between GAS use and time to first episode of all-cause peritonitis were examined using Cox proportional hazards models. The primary exposure of interest was GAS and secondarily PPI or H2RA use. Secondary outcomes were organism-specific peritonitis, peritonitis cure rates, and death.
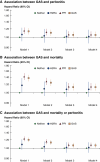
Results: Among patients (N=23,797) at study baseline, 6020 (25.3%) used PPIs, and 1382 (5.8%) used H2RAs. Overall risks of GAS use and peritonitis risk (adjusted hazard ratio [AHR]=1.05, 95% confidence interval [CI], 0.98 to 1.13]) and use of PPI (AHR 1.06 [95% CI, 0.99 to 1.14]) or H2RA (AHR 1.02 [95% CI, 0.88 to 1.18]) did not reach statistical significance. In organism-specific analyses, GAS users displayed higher peritonitis risks for Gram-negative (AHR 1.29, 95% CI, 1.05 to 1.57), Gram-positive (AHR 1.15, 95% CI, 1.01 to 1.31), culture-negative (AHR 1.20, 95% CI, 1.01 to 1.42), enteric (AHR 1.23, 95% CI, 1.03 to 1.48), and particularly Streptococcal (AHR 1.47, 95% CI, 1.15 to 1.89) peritonitis episodes. GAS was also associated with higher overall mortality (AHR 1.13 [95% CI, 1.05 to 1.22]).
Conclusion: The association between GAS use and peritonitis risk was weaker (hazard ratio [HR] 1.05 [0.98 to 1.13]) than for streptococcal (HR 1.57 [1.15 to 1.89]) and Gram-negative (HR 1.29 [1.05 to 1.57]) peritonitis. A better understanding of mechanisms surrounding the differential effects of GAS subtype on peritonitis risks is needed. Clinicians should be cautious when prescribing GAS. The impact of GAS deprescribing on peritonitis risk requires further evaluation.
Conflict of interest statement
B. Bieber reports the following: Research Funding: Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see
Figures



References
-
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2–3. doi:10.1136/bmj.39406.449456.BE - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

