Engineering CD276/B7-H3-targeted antibody-drug conjugates with enhanced cancer-eradicating capability
- PMID: 38019654
- PMCID: PMC10872261
- DOI: 10.1016/j.celrep.2023.113503
Engineering CD276/B7-H3-targeted antibody-drug conjugates with enhanced cancer-eradicating capability
Abstract
CD276/B7-H3 represents a promising target for cancer therapy based on widespread overexpression in both cancer cells and tumor-associated stroma. In previous preclinical studies, CD276 antibody-drug conjugates (ADCs) exploiting a talirine-type pyrrolobenzodiazepine (PBD) payload showed potent activity against various solid tumors but with a narrow therapeutic index and dosing regimen higher than that tolerated in clinical trials using other antibody-talirine conjugates. Here, we describe the development of a modified talirine PBD-based fully human CD276 ADC, called m276-SL-PBD, that is cross-species (human/mouse) reactive and can eradicate large 500-1,000-mm3 triple-negative breast cancer xenografts at doses 10- to 40-fold lower than the maximum tolerated dose. By combining CD276 targeting with judicious genetic and chemical ADC engineering, improved ADC purification, and payload sensitivity screening, these studies demonstrate that the therapeutic index of ADCs can be substantially increased, providing an advanced ADC development platform for potent and selective targeting of multiple solid tumor types.
Keywords: ADC; B7-H3; B7H3; CD276; CP: Cancer; PBD; TNBC; antibody-drug conjugate; breast cancer; m276-SL-PBD; pyrrolobenzodiazepine.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests Y.F., Z.Z., D.S.D., and B.S.C. are inventors of intellectual property related to m276 antibody-drug conjugates.
Figures

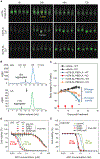



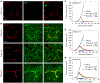

References
-
- Beck A, Goetsch L, Dumontet C, and Corvaïa N (2017). Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov 16, 315–337. - PubMed
-
- Amiri-Kordestani L, Blumenthal GM, Xu QC, Zhang L, Tang SW, Ha L, Weinberg WC, Chi B, Candau-Chacon R, Hughes P, et al. (2014). FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin. Cancer Res 20, 4436–4441. - PubMed
-
- Fuentes-Antrás J, Genta S, Vijenthira A, and Siu LL (2023). Antibody-drug conjugates: in search of partners of choice. Trends Cancer 9, 339–354. - PubMed
-
- Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao NS, Tice DA, and Soria JC (2019). Antibody-Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res 25, 5441–5448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

