GDF8 inhibition enhances musculoskeletal recovery and mitigates posttraumatic osteoarthritis following joint injury
- PMID: 38019905
- PMCID: PMC10686569
- DOI: 10.1126/sciadv.adi9134
GDF8 inhibition enhances musculoskeletal recovery and mitigates posttraumatic osteoarthritis following joint injury
Abstract
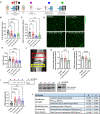
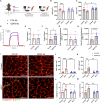
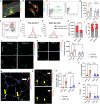
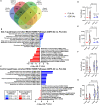
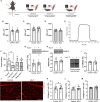
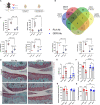
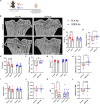
Musculoskeletal disorders contribute substantially to worldwide disability. Anterior cruciate ligament (ACL) tears result in unresolved muscle weakness and posttraumatic osteoarthritis (PTOA). Growth differentiation factor 8 (GDF8) has been implicated in the pathogenesis of musculoskeletal degeneration following ACL injury. We investigated GDF8 levels in ACL-injured human skeletal muscle and serum and tested a humanized monoclonal GDF8 antibody against a placebo in a mouse model of PTOA (surgically induced ACL tear). In patients, muscle GDF8 was predictive of atrophy, weakness, and periarticular bone loss 6 months following surgical ACL reconstruction. In mice, GDF8 antibody administration substantially mitigated muscle atrophy, weakness, and fibrosis. GDF8 antibody treatment rescued the skeletal muscle and articular cartilage transcriptomic response to ACL injury and attenuated PTOA severity and deficits in periarticular bone microarchitecture. Furthermore, GDF8 genetic deletion neutralized musculoskeletal deficits in response to ACL injury. Our findings support an opportunity for rapid targeting of GDF8 to enhance functional musculoskeletal recovery and mitigate the severity of PTOA after injury.
Figures
References
-
- C. J. Murray, C. Atkinson, K. Bhalla, G. Birbeck, R. Burstein, D. Chou, R. Dellavalle, G. Danaei, M. Ezzati, A. Fahimi, D. Flaxman, S. Foreman, E. Gabriel, N. Gakidou, S. Kassebaum, S. Khatibzadeh, S. Lim, E. Lipshultz, S. London, M. Lopez, F. MacIntyre, A. H. Mokdad, A. Moran, A. E. Moran, D. Mozaffarian, T. Murphy, M. Naghavi, C. Pope, T. Roberts, J. Salomon, D. C. Schwebel, S. Shahraz, D. A. Sleet, J. Murray, M. Abraham, K. Ali, D. H. Bartels, H. Chen, M. H. Criqui, J. Dahodwala, E. L. Ding, E. R. Dorsey, B. E. Ebel, S. Fahami, A. Flaxman, D. Flaxman, D. Gonzalez-Medina, B. Grant, H. Hagan, H. Hoffman, J. L. Leasher, J. Lin, R. Lozano, Y. Lu, L. Mallinger, M. M. McDermott, R. Micha, T. R. Miller, A. A. Mokdad, K. M. Narayan, S. B. Omer, P. M. Pelizzari, D. Phillips, D. Ranganathan, F. P. Rivara, U. Sampson, E. Sanman, A. Sapkota, S. Sharaz, R. Shivakoti, G. M. Singh, D. Singh, M. Tavakkoli, J. A. Towbin, J. D. Wilkinson, A. Zabetian, M. Alvardo, L. M. Baddour, E. J. Benjamin, I. Bolliger, E. Carnahan, S. S. Chugh, A. Cohen, K. E. Colson, L. T. Cooper, W. Couser, K. C. Dabhadkar, R. P. Dellavalle, D. Dicker, H. Duber, R. E. Engell, D. T. Felson, M. M. Finucane, T. Fleming, M. H. Forouzanfar, G. Freedman, M. K. Freeman, R. F. Gillum, R. Gosselin, H. R. Gutierrez, R. Havmoeller, K. H. Jacobsen, S. L. James, R. Jasrasaria, S. Jayarman, N. Johns, Q. Lan, M. Meltzer, G. A. Mensah, C. Michaud, C. Mock, T. E. Moffitt, R. G. Nelson, C. Olives, K. Ortblad, B. Ostro, M. Raju, H. Razavi, B. Ritz, R. L. Sacco, K. Shibuya, D. Silberberg, J. A. Singh, K. Steenland, J. A. Taylor, G. D. Thurston, M. S. Vavilala, T. Vos, G. R. Wagner, M. A. Weinstock, M. G. Weisskopf, S. Wulf; U.S. Burden of Disease Collaborators , The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA 310, 591–606 (2013). - PMC - PubMed
-
- H. Kotlarz, C. L. Gunnarsson, H. Fang, J. A. Rizzo, Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum. 60, 3546–3553 (2009). - PubMed
-
- T. D. Brown, R. C. Johnston, C. L. Saltzman, J. L. Marsh, J. A. Buckwalter, Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 20, 739–744 (2006). - PubMed
-
- L. Y. Griffin, M. J. Albohm, E. A. Arendt, R. Bahr, B. D. Beynnon, M. Demaio, R. W. Dick, L. Engebretsen, W. E. Garrett Jr., J. A. Hannafin, T. E. Hewett, L. J. Huston, M. L. Ireland, R. J. Johnson, S. Lephart, B. R. Mandelbaum, B. J. Mann, P. H. Marks, S. W. Marshall, G. Myklebust, F. R. Noyes, C. Powers, C. Shields Jr., S. J. Shultz, H. Silvers, J. Slauterbeck, D. C. Taylor, C. C. Teitz, E. M. Wojtys, B. Yu, Understanding and preventing noncontact anterior cruciate ligament Injuries. Am. J. Sports Med. 34, 1512–1532 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

