A T cell receptor β chain-directed antibody fusion molecule activates and expands subsets of T cells to promote antitumor activity
- PMID: 38019931
- PMCID: PMC11421222
- DOI: 10.1126/scitranslmed.adi0258
A T cell receptor β chain-directed antibody fusion molecule activates and expands subsets of T cells to promote antitumor activity
Abstract
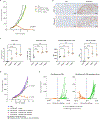
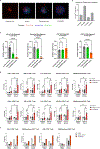
Despite the success of programmed cell death-1 (PD-1) and PD-1 ligand (PD-L1) inhibitors in treating solid tumors, only a proportion of patients respond. Here, we describe a first-in-class bifunctional therapeutic molecule, STAR0602, that comprises an antibody targeting germline Vβ6 and Vβ10 T cell receptors (TCRs) fused to human interleukin-2 (IL-2) and simultaneously engages a nonclonal mode of TCR activation with costimulation to promote activation and expansion of αβ T cell subsets expressing distinct variable β (Vβ) TCR chains. In solution, STAR0602 binds IL-2 receptors in cis with Vβ6/Vβ10 TCRs on the same T cell, promoting expansion of human Vβ6 and Vβ10 CD4+ and CD8+ T cells that acquire an atypical central memory phenotype. Monotherapy with a mouse surrogate molecule induced durable tumor regression across six murine solid tumor models, including several refractory to anti-PD-1. Analysis of murine tumor-infiltrating lymphocyte (TIL) transcriptomes revealed that expanded Vβ T cells acquired a distinct effector memory phenotype with suppression of genes associated with T cell exhaustion and TCR signaling repression. Sequencing of TIL TCRs also revealed an increased T cell repertoire diversity within targeted Vβ T cell subsets, suggesting clonal revival of tumor T cell responses. These immunological and antitumor effects in mice were recapitulated in studies of STAR0602 in nonhuman primates and human ex vivo models, wherein STAR0602 boosted human antigen-specific T cell responses and killing of tumor organoids. Thus, STAR0602 represents a distinct class of T cell-activating molecules with the potential to deliver enhanced antitumor activity in checkpoint inhibitor-refractory settings.
Conflict of interest statement
JH, MK, AB, JL, WH, GG, KS, JT, RS, RC, KL, and ZS are employees of Marengo Therapeutics and own stock in the company. AS has a consulting agreement in place with Marengo Therapeutics. RND, YTT, JS, and JLG report that their institute (NCI) has a Cooperative Research and Development Agreement (CRADA) with Marengo Therapeutics. JH, MK, GG and SP are inventors on patent applications US63/172,468 and PCT/US2022/023922 held/submitted by Marengo Therapeutics that cover anti-TCRVβ x Cytokine. EJW is a member of the Parker Institute for Cancer Immunotherapy and has consulting agreements with or is on the scientific advisory board for Merck, Roche, Pieris, Marengo Therapeutics and Surface Oncology. EJW has a patent licensing agreement on the PD-1 pathway with Roche–Genentech and is a founder of Arsenal Biosciences. J.L.G. as part of his official duties is an uncompensated co-chair of the SAB for Marengo.
Figures






References
-
- Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, Noppeney R, Hess G, Kallert S, Mackensen A, Rupertus K, Kanz L, Libicher M, Nagorsen D, Zugmaier G, Klinger M, Wolf A, Dorsch B, Quednau BD, Schmidt M, Scheele J, Baeuerle PA, Leo E, Bargou RC, Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J Clin Oncol 34, 1104–1111 (2016). - PubMed
-
- Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Puré E, Milone MC, June CH, Riley JL, Wherry EJ, Albelda SM, Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 20, 4262–4273 (2014). - PMC - PubMed
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366, 2443–2454 (2012). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

