Comparison of automated subcutaneous defibrillator screening between different pacing sites in cardiac pacing device carriers
- PMID: 38019960
- PMCID: PMC10751811
- DOI: 10.1093/europace/euad352
Comparison of automated subcutaneous defibrillator screening between different pacing sites in cardiac pacing device carriers
Abstract
Aims: The compatibility of cardiac pacing with the presence of a subcutaneous implantable cardioverter-defibrillator (S-ICD) has been investigated, but S-ICD screening test results have not been compared among different pacing sites. The objective was to compare S-ICD screening results among different cardiac pacing sites and to assess the electrocardiographic predictors of success.
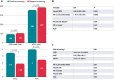
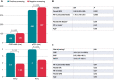
Methods and results: This prospective single-centre study conducted automated S-ICD screening in 102 carriers of cardiac pacing devices in conduction system (CSP), biventricular (BVP), right ventricular outflow tract (RVOT), or right ventricular apex (RVA) pacing sites. The study included 102 patients: 40 with CSP (20 left bundle pacing and 20 His bundle pacing), 21 with BVP, and 20 and 21 with RVOT and RVA pacing, respectively. The percentage of positive screenings was significantly higher for CSP (97.5%) than for the other patient groups (BVP 71.4%, RVOT 70%, and RVA 19%). In multivariate analysis, positive screening was associated with a narrower QRS (OR 0.95 [0.92-0.98] P = 0.001) and higher R/T ratio in precordial leads (1.76 [1.18-2.61]).
Conclusion: A higher S-ICD eligibility rate of cardiac pacing device carriers was obtained in CSP than in conventional pacing (RVA or RVOT) or BVP. The presence of narrower paced QRS width and paced corrected QT interval and of higher R/T ratio in precordial and limb leads are electrocardiographic predictors of a positive response to screening.
Keywords: Conduction system pacing; His bundle pacing; Left bundle pacing; Screening; Subcutaneous defibrillator.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared.
Figures
Comment on
-
The need for a subsequent transvenous system in patients implanted with subcutaneous implantable cardioverter-defibrillator.Heart Rhythm. 2022 Dec;19(12):1958-1964. doi: 10.1016/j.hrthm.2022.06.030. Epub 2022 Jun 30. Heart Rhythm. 2022. PMID: 35781042
References
-
- Monkhouse C, Wharmby A, Carter Z, Hunter R, Dhinoja M, Chow A et al. Exploiting SMART pass filter deactivation detection to minimize inappropriate subcutaneous implantable cardioverter defibrillator therapies: a real-world single-centre experience and management guide. Europace 2023;25:euad040. - PMC - PubMed
-
- Olde Nordkamp LR, Brouwer TF, Barr C, Theuns DA, Boersma LV, Johansen JB et al. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol 2015;195:126–33. - PubMed
-
- Hakamata T, Otsuki S, Izumi D, Sakaguchi Y, Suzuki N, Ikami Y et al. Clinical impact of ECG changes on oversensing of subcutaneous implantable cardioverter-defibrillators. Heart Rhythm 2022;19:1704–11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

