Study of the electronic effect and quantitative spectra predictions of o-methoxyaniline-terminated monoazonaphthols: a combined experimental and DFT study
- PMID: 38020020
- PMCID: PMC10655066
- DOI: 10.1039/d3ra05518c
Study of the electronic effect and quantitative spectra predictions of o-methoxyaniline-terminated monoazonaphthols: a combined experimental and DFT study
Abstract
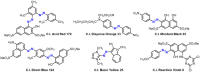
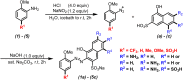
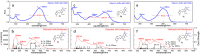
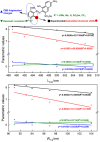
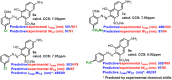
A combined experimental and density functional theory (DFT) study on the UV-Vis spectra of o-methoxyaniline-terminated mono azo dyes was conducted. By applying time-dependent-DFT calculations, details of excitation processes were determined and visualization by hole-electron analysis was undertaken. Fragment-divided analysis revealed the contributions of different parts of the structures for the UV-Vis spectra, that richer/poorer electron density on aromatic rings lead to greater/less maximum absorption wavelengths (λmax) and larger/smaller half peak width (W1/2). Combining theoretical prediction with experimental verification, we answered the question of how the electronegativities of substituents affected the electron densities and how it affected the spectra. In addition, a linear model connecting the λmax and W1/2 to the chemical shifts obtained by NMR spectroscopy was constructed, which laid the foundation for construction of a spectral library.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


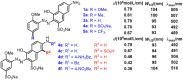
Similar articles
-
Synthesis of Some New Heterocyclic Azo Dyes Derived from 2-amino-3-cyano-4.6- diarylpyridines and Investigation of its Absorption Spectra and Stability using the DFT.Curr Org Synth. 2021;18(5):506-516. doi: 10.2174/1570179417666201218164519. Curr Org Synth. 2021. PMID: 33342417
-
Synthesis, electronic, and spectral properties of novel geranylated chalcone derivatives: a theoretical and experimental study.J Mol Model. 2016 Oct;22(10):253. doi: 10.1007/s00894-016-3114-x. Epub 2016 Oct 3. J Mol Model. 2016. PMID: 27699552
-
DFT calculations and experimental FT-IR, FT-Raman, NMR, UV-Vis spectral studies of 3-fluorophenylboronic acid.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136 Pt B:306-20. doi: 10.1016/j.saa.2014.08.141. Epub 2014 Oct 5. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25448934
-
Theoretical insights into UV-Vis absorption spectra of difluoroboron β-diketonates with an extended π system: An analysis based on DFT and TD-DFT calculations.Spectrochim Acta A Mol Biomol Spectrosc. 2019 Jun 5;216:161-172. doi: 10.1016/j.saa.2019.02.064. Epub 2019 Feb 27. Spectrochim Acta A Mol Biomol Spectrosc. 2019. PMID: 30897377
-
Experimental and theoretical (FT-IR, FT-Raman, UV-vis, NMR) spectroscopic analysis and first order hyperpolarizability studies of non-linear optical material: (2E)-3-[4-(methylsulfanyl) phenyl]-1-(4-nitrophenyl) prop-2-en-1-one using density functional theory.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Sep 15;130:41-53. doi: 10.1016/j.saa.2014.03.072. Epub 2014 Apr 8. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24762572
Cited by
-
Photovoltaic Performance of Azo-Benzaldehyde Sensitizers in DSSCs: A DFT-Experimental Correlation.J Fluoresc. 2025 Aug 26. doi: 10.1007/s10895-025-04517-2. Online ahead of print. J Fluoresc. 2025. PMID: 40856731
References
-
- Bafana A. Devi S. S. Chakrabarti T. Environ. Rev. 2011;19:350–370.
-
- Chavan R. B., Handbook of Textile and Industrial Dyeing (in Woodhead Publishing Series in Textiles). Principles, Processes and Types of Dyes, 2011, vol. 1, pp. 515–561
-
-
For selected papers on spectra studies and dyes designing, see:
- Benkhaya S. M' rabet S. El Harfi A. Inorg. Chem. Commun. 2020;115:107891.
- Shindy H. A. Dyes Pigm. 2017;145:505–513.
- Shahab S. Hajikolaee F. H. Filippovich L. Darroudi M. Loiko V. A. Kumar R. Borzehandani M. Y. Dyes Pigm. 2016;129:9–17.
-
-
-
For selected patents or papers on redshift or blueshift control of dyes, see:
- Zhu G., Jiang J., Li Z., Song H. and Zeng Y., 2022, CN114539803A
- Chen B. Ni S. Sun L. Luo X. Zhang Q. Song Y. Zhong Q. Fang Y. Huang C. Chen S. Wu W. Dyes Pigm. 2018;158:474–481.
-
-
-
For selected papers on recent applications of dyes, see:
- Gong J. Sumathy K. Qiao Q. Zhou Z. Renewable Sustainable Energy Rev. 2017;68:234–246.
- Richhariya G. Kumar A. Tekasakul P. Gupta B. Renewable Sustainable Energy Rev. 2017;69:705–718.
- Kim K.-H. Lee S. Moon C.-K. Kim S.-Y. Park Y.-S. Lee J.-H. Lee J. W. Huh J. You Y. Kim J.-J. Nat. Comm. 2014;5:4769. - PubMed
- Chang J. B. Hwang J. H. Park J. S. Kim J. P. Dyes Pigm. 2011;88(3):366–371.
- Mochizuki N. Ishinabe T. Fujiwara D. Nakamura D. Koma N. Fujikake H. ITE Trans. Media Technol. Appl. 2018;6(4):262–268.
- Nobuhiro K. Keita F. Chem. Lett. 2005;34:558–559.
-
LinkOut - more resources
Full Text Sources

