Novel styryl-thiazole hybrids as potential anti-Alzheimer's agents
- PMID: 38020070
- PMCID: PMC10650344
- DOI: 10.1039/d3md00308f
Novel styryl-thiazole hybrids as potential anti-Alzheimer's agents
Abstract
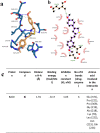
In this study, combining the thiazole and cinnamoyl groups into the styryl-thiazole scaffold, a series of novel styryl-thiazole hybrids (6a-p) was rationally designed, synthesized, and evaluated by the multi-target-directed ligands strategy as potential candidates for the treatment of Alzheimer's disease (AD). Hybrids 6e and 6i are the most promising among the synthesized hybrids since they are able to significantly increase cell viabilities in Aβ1-42-exposed-human neuroblastoma cell line (6i at the concentration of 50 μg mL-1 and 6e at the concentration of 25 μg mL-1 resulted in ∼34% and ∼30% increase in cell viabilities, respectively). Compounds 6e and 6i exhibit highly AChE inhibitory properties in the experimental AD model at 375.6 ± 18.425 mU mL-1 and 397.6 ± 32.152 mU mL-1, respectively. Moreover, these data were also confirmed by docking studies and in vitro enzyme inhibition assays. Compared to hybrid 6e and according to the results, 6i also has the highest potential against Aβ1-42 aggregation with over 80% preventive activity. The in silico prediction of the physicochemical properties confirms that 6i possesses a better profile compared to 6e. Therefore, compound 6i presents a promising multi-targeted active molecular profile for treating AD considering the multifactorial nature of AD, and it is reasonable to deepen its mechanisms of action in an in vivo experimental model of AD.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Discovery of new phenyl sulfonyl-pyrimidine carboxylate derivatives as the potential multi-target drugs with effective anti-Alzheimer's action: Design, synthesis, crystal structure and in-vitro biological evaluation.Eur J Med Chem. 2021 Apr 5;215:113224. doi: 10.1016/j.ejmech.2021.113224. Epub 2021 Feb 2. Eur J Med Chem. 2021. PMID: 33582578
-
Design, synthesis and neuroprotective evaluation of novel tacrine-benzothiazole hybrids as multi-targeted compounds against Alzheimer's disease.Bioorg Med Chem. 2013 Aug 1;21(15):4559-69. doi: 10.1016/j.bmc.2013.05.028. Epub 2013 May 24. Bioorg Med Chem. 2013. PMID: 23768661
-
Combined in Vitro and in Silico Studies for the Anticholinesterase Activity and Pharmacokinetics of Coumarinyl Thiazoles and Oxadiazoles.Front Chem. 2018 Mar 26;6:61. doi: 10.3389/fchem.2018.00061. eCollection 2018. Front Chem. 2018. PMID: 29632858 Free PMC article.
-
Multi-target-directed ligands in Alzheimer's disease treatment.Curr Med Chem. 2011;18(32):4949-75. doi: 10.2174/092986711797535245. Curr Med Chem. 2011. PMID: 22050745 Review.
-
Rational drug design strategies for the development of promising multi-target directed indole hybrids as Anti-Alzheimer agents.Bioorg Chem. 2022 Oct;127:105941. doi: 10.1016/j.bioorg.2022.105941. Epub 2022 Jun 10. Bioorg Chem. 2022. PMID: 35714473 Review.
Cited by
-
Recent Advances in the Search for Effective Anti-Alzheimer's Drugs.Int J Mol Sci. 2024 Dec 27;26(1):157. doi: 10.3390/ijms26010157. Int J Mol Sci. 2024. PMID: 39796014 Free PMC article. Review.
-
Facile One-Pot Fischer-Suzuki-Knoevenagel Microwave-Assisted Synthesis of Fluorescent 5-Aryl-2-Styryl-3H-Indoles.Molecules. 2025 Jun 7;30(12):2503. doi: 10.3390/molecules30122503. Molecules. 2025. PMID: 40572469 Free PMC article.
References
-
- Ham Y. H. Jason Chan K. K. Chan W. Chem. Res. Toxicol. 2020;33:1815–1821. - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials

