Organotypic heterogeneity in microvascular endothelial cell responses in sepsis-a molecular treasure trove and pharmacological Gordian knot
- PMID: 38020105
- PMCID: PMC10665520
- DOI: 10.3389/fmed.2023.1252021
Organotypic heterogeneity in microvascular endothelial cell responses in sepsis-a molecular treasure trove and pharmacological Gordian knot
Abstract
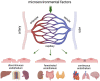
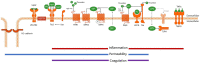
In the last decades, it has become evident that endothelial cells (ECs) in the microvasculature play an important role in the pathophysiology of sepsis-associated multiple organ dysfunction syndrome (MODS). Studies on how ECs orchestrate leukocyte recruitment, control microvascular integrity and permeability, and regulate the haemostatic balance have provided a wealth of knowledge and potential molecular targets that could be considered for pharmacological intervention in sepsis. Yet, this information has not been translated into effective treatments. As MODS affects specific vascular beds, (organotypic) endothelial heterogeneity may be an important contributing factor to this lack of success. On the other hand, given the involvement of ECs in sepsis, this heterogeneity could also be leveraged for therapeutic gain to target specific sites of the vasculature given its full accessibility to drugs. In this review, we describe current knowledge that defines heterogeneity of organ-specific microvascular ECs at the molecular level and elaborate on studies that have reported EC responses across organ systems in sepsis patients and animal models of sepsis. We discuss hypothesis-driven, single-molecule studies that have formed the basis of our understanding of endothelial cell engagement in sepsis pathophysiology, and include recent studies employing high-throughput technologies. The latter deliver comprehensive data sets to describe molecular signatures for organotypic ECs that could lead to new hypotheses and form the foundation for rational pharmacological intervention and biomarker panel development. Particularly results from single cell RNA sequencing and spatial transcriptomics studies are eagerly awaited as they are expected to unveil the full spatiotemporal signature of EC responses to sepsis. With increasing awareness of the existence of distinct sepsis subphenotypes, and the need to develop new drug regimen and companion diagnostics, a better understanding of the molecular pathways exploited by ECs in sepsis pathophysiology will be a cornerstone to halt the detrimental processes that lead to MODS.
Keywords: -omics; animal models; biomarkers; endothelial cell heterogeneity; human studies; multiple organ dysfunction syndrome; pharmacology; sepsis.
Copyright © 2023 Cleuren and Molema.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

