Flexibility in zeolites: origin, limits, and evaluation
- PMID: 38020361
- PMCID: PMC10646982
- DOI: 10.1039/d3sc03934j
Flexibility in zeolites: origin, limits, and evaluation
Abstract
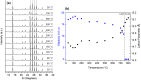
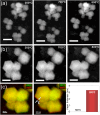
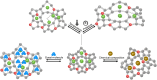
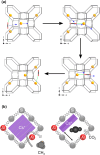
Numerous pieces of evidence in the literature suggest that zeolitic materials exhibit significant intrinsic flexibility as a consequence of the spring-like behavior of Si-O and Al-O bonds and the distortion ability of Si-O-Si and Al-O-Si angles. Understanding the origin of flexibility and how it may be tuned to afford high adsorption selectivity in zeolites is a big challenge. Zeolite flexibility may be triggered by changes in temperature, pressure, or chemical composition of the framework and extra-framework compounds, as well as by the presence of guest molecules. Therefore, zeolite flexibility can be classified into three categories: (i) temperature and pressure-induced flexibility; (ii) guest-induced flexibility; and (iii) compositionally-induced flexibility. An outlook on zeolite flexibility and the challenges met during the precise experimental evaluations of zeolites will be discussed. Overcoming these challenges will provide an important tool for designing novel selective adsorbents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Ghojavand S. Coasne B. Clatworthy E. B. Guillet-Nicolas R. Bazin P. Desmurs M. Jacobo Aguilera L. Ruaux V. Mintova S. Alkali Metal Cations Influence the CO2 Adsorption Capacity of Nanosized Chabazite: Modeling vs. Experiment. ACS Appl. Nano Mater. 2022;5(4):5578–5588. doi: 10.1021/acsanm.2c00537. doi: 10.1021/acsanm.2c00537. - DOI - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

