Benzoyldiisopropylchlorosilane: a visible light photocleavable alcohol protecting group
- PMID: 38020376
- PMCID: PMC10646889
- DOI: 10.1039/d3sc04975b
Benzoyldiisopropylchlorosilane: a visible light photocleavable alcohol protecting group
Abstract
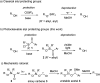
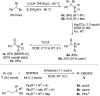
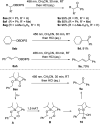
Silyl chlorides are highly valuable and popular reagents for the protection of alcohols. In this edge article we introduce a photocleavable alcohol protecting group on the basis of acyl silanes. To achieve this, acylchlorosilanes that represent a new class of acylsilanes were developed. They can be easily synthesized in a concise sequence of three steps in high overall yield. Alcohol silyl protection takes place under established mild conditions, akin to those associated with classical silicon-based protecting groups. The removal of the Si-group is achieved at room temperature through exposure to visible light (456 nm) in methanol. We demonstrate a broad spectrum of substrates with remarkable tolerance toward diverse functional groups, highlighting a substantial level of orthogonality with respect to other protecting groups. Furthermore, we showcase the robustness of this approach against various transformations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Greene T. W. and Wuts P. G. M., Protective Groups in Organic Synthesis, 3rd edn, J. Wiley, New York, 1999
- Kocieński P. J., Protecting Groups, Thieme, Stuttgart, 1994
- Schelhaas M. Waldmann H. Angew. Chem. Int. Ed. 1996;35:2056. doi: 10.1002/anie.199620561. - DOI
-
-
Selected examples:
- Armstrong R. W. Beau J.-M. Cheon S. H. Christ W. J. Fujioka H. Ham W.-H. Hawkins L. D. Jin H. Kang S. H. Kishi Y. Martinelli M. J. McWhorter Jr W. W. Mizuno M. Nakata M. Stutz A. E. Talamas F. X. Taniguchi M. Tino J. A. Ueda K. Uenishi J. White J. B. Yonag M. J. Am. Chem. Soc. 1989;111:7530. doi: 10.1021/ja00201a038. - DOI
- Kamenecka T. M. Danishefsky S. J. Angew. Chem. Int. Ed. 1998;37:2995. doi: 10.1002/(SICI)1521-3773(19981116)37:21<2995::AID-ANIE2995>3.0.CO;2-6. - DOI - PubMed
-
-
- Corey E. J. Venkateswarlu A. J. Am. Chem. Soc. 1972;94:6190. doi: 10.1021/ja00772a043. - DOI
LinkOut - more resources
Full Text Sources

