Donor-Derived Cell-Free DNA (dd-cfDNA) in Kidney Transplant Recipients With Indication Biopsy-Results of a Prospective Single-Center Trial
- PMID: 38020751
- PMCID: PMC10654198
- DOI: 10.3389/ti.2023.11899
Donor-Derived Cell-Free DNA (dd-cfDNA) in Kidney Transplant Recipients With Indication Biopsy-Results of a Prospective Single-Center Trial
Abstract
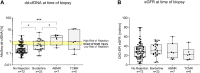
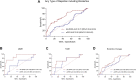
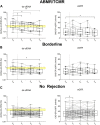
Donor-derived cell-free DNA (dd-cfDNA) identifies allograft injury and discriminates active rejection from no rejection. In this prospective study, 106 kidney transplant recipients with 108 clinically indicated biopsies were enrolled at Heidelberg University Hospital between November 2020 and December 2022 to validate the clinical value of dd-cfDNA in a cohort of German patients. dd-cfDNA was quantified at biopsy and correlated to histopathology. Additionally, dd-cfDNA was determined on days 7, 30, and 90 post-biopsy and analyzed for potential use to monitor response to anti-rejection treatment. dd-cfDNA levels were with a median (IQR) % of 2.00 (0.48-3.20) highest in patients with ABMR, followed by 0.92 (0.19-11.25) in patients with TCMR, 0.44 (0.20-1.10) in patients with borderline changes and 0.20 (0.11-0.53) in patients with no signs of rejection. The AUC for dd-cfDNA to discriminate any type of rejection including borderline changes from no rejection was at 0.72 (95% CI 0.62-0.83). In patients receiving anti-rejection treatment, dd-cfDNA levels significantly decreased during the 7, 30, and 90 days follow-up compared to levels at the time of biopsy (p = 0.006, p = 0.002, and p < 0.001, respectively). In conclusion, dd-cfDNA significantly discriminates active rejection from no rejection. Decreasing dd-cfDNA following anti-rejection treatment may indicate response to therapy. Clinical Trial Registration: https://drks.de/search/de/trial/DRKS00023604, identifier DRKS00023604.
Keywords: dd-cfDNA; donor-derived cell-free DNA; kidney transplantation; rejection; response to therapy.
Copyright © 2023 Benning, Morath, Fink, Rudek, Speer, Kälble, Nusshag, Beimler, Schwab, Waldherr, Zeier, Süsal and Tran.
Conflict of interest statement
This study received funding from CareDx Inc. (Brisbane, CA). The funder had the following involvement with the study: funding for kits and supplies for dd-cfDNA testing.
Figures
Comment in
-
Donor-Derived Cell-Free DNA: Attractive Biomarker Seeks a Context of Use.Transpl Int. 2023 Dec 1;36:12406. doi: 10.3389/ti.2023.12406. eCollection 2023. Transpl Int. 2023. PMID: 38106814 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

