Objective assessment of eye alignment and disparity-driven vergence in Parkinson's disease
- PMID: 38020777
- PMCID: PMC10643751
- DOI: 10.3389/fnagi.2023.1217765
Objective assessment of eye alignment and disparity-driven vergence in Parkinson's disease
Abstract
Background: Self-reported diplopia is described in up to one-third of Parkinson's disease (PD) patients.
Objective: The purpose of our study was to expand our understanding of the mechanistic underpinnings of diplopia in PD. We hypothesize that the time-based control of eye alignment and increased eye deviation under binocular viewing will be related to the fusion-initiating and fusion-maintaining component deficits of disparity-driven vergence in PD.
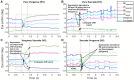
Methods: We used high-resolution video-oculography to measure eye alignment under binocular and monocular viewing and disparity-driven vergence in 33 PD and 10 age-matched healthy participants. We computed eye deviation and time-based control of eye alignment, occurrence of conjugate saccadic eye movements, latency and gain of vergence (fusion initiation), and variance of eye position at the end of dynamic vergence (fusion maintenance).
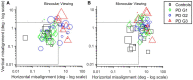
Results: We categorized PD subjects into three groups, considering their time-based control of eye alignment as compared to healthy controls in binocular viewing. Group 1 = 45% had good control and spent >80% of the time when the eyes were well-aligned, Group 2 = 26% had intermediate control and spent <80% but greater >5% of the time when the eyes were well-aligned, and Group 3 = 29% had very poor control with increased eye deviation majority of the times (<5% of the time when the eyes were well-aligned). All three groups exhibited greater eye deviation under monocular viewing than controls. PD subjects exhibited fusion-initiating and fusion-maintaining vergence deficits (prolonged latencies, reduced vergence gain, increased variance of fusion-maintaining component) with a greater probability of saccadic movements than controls. Group 2 and Group 3 subjects were more likely to exhibit failure to initiate vergence (>20%) than Group 1 (13%) and controls (0%) trials. No significant difference was found in the Unified Parkinson's Disease Rating Scale (UPDRS-a tool to measure the severity of PD) values between the three PD groups (Group 1 = 33.69 ± 14.22, Group 2 = 38.43 ± 22.61, and Group 3 = 23.44 ± 1, p > 0.05).
Conclusion: The majority of PD subjects within our cohort had binocular dysfunction with increased eye deviation under monocular viewing and disparity-driven vergence deficits. PD subjects with intermediate or poor control of eye deviation under binocular viewing had greater fusion-initiating and fusion-maintaining vergence deficits. The study highlights the importance of assessing binocular dysfunction in PD subjects independent of the severity of motor symptoms.
Keywords: Parkinson’s disease; basal ganglia; density-based clustering; strabismus; supra-oculomotor area; vergence.
Copyright © 2023 Gupta, Murray, Beylergil, Jacobs, Kilbane, Shaikh and Ghasia.
Conflict of interest statement
The author(s) FG declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

