Not all (cells) who wander are lost: Upstream migration as a pervasive mode of amoeboid cell motility
- PMID: 38020916
- PMCID: PMC10651737
- DOI: 10.3389/fcell.2023.1291201
Not all (cells) who wander are lost: Upstream migration as a pervasive mode of amoeboid cell motility
Abstract
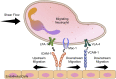
Leukocytes possess the ability to migrate upstream-against the direction of flow-on surfaces of specific chemistry. Upstream migration was first characterized in vitro for T-cells on surfaces comprised of intracellular adhesion molecule-1 (ICAM-1). Upstream migration occurs when the integrin receptor αLβ2 (also known as lymphocyte function-associated antigen-1, or LFA-1) binds to ICAM-1. LFA-1/ICAM-1 interactions are ubiquitous and are widely found in leukocyte trafficking. Upstream migration would be employed after cells come to arrest on the apical surface of the endothelium and might confer an advantage for both trans-endothelial migration and tissue surveillance. It has now been shown that several other motile amoeboid cells which have the responsibility of trafficking from blood vessels into tissues, such as Marginal zone B cells, hematopoietic stem cells, and neutrophils (when macrophage-1 antigen, Mac-1, is blocked), can also migrate upstream on ICAM-1 surfaces. This review will summarize what is known about the basic mechanisms of upstream migration, which cells have displayed this phenomenon, and the possible role of upstream migration in physiology and tissue homeostasis.
Keywords: ICAM-1; LFA-1; T-cells; hematopoietic stem cells; inflammation; leukocytes; migration.
Copyright © 2023 Buffone, Hammer, Kim, Anderson, Mochida, Lee and Guin.
Conflict of interest statement
NA is a current employee of Carisma Therapeutics (Philadelphia, PA). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- AbuSamra D. B., Aleisa F. A., Al-Amoodi A. S., Jalal Ahmed H. M., Chin C. J., Abuelela A. F., et al. (2017). Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 1 (27), 2799–2816. 10.1182/bloodadvances.2017004317 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

