Oral and Topical Administration of a Standardized Saw Palmetto Oil Reduces Hair Fall and Improves the Hair Growth in Androgenetic Alopecia Subjects - A 16-Week Randomized, Placebo-Controlled Study
- PMID: 38021422
- PMCID: PMC10648974
- DOI: 10.2147/CCID.S435795
Oral and Topical Administration of a Standardized Saw Palmetto Oil Reduces Hair Fall and Improves the Hair Growth in Androgenetic Alopecia Subjects - A 16-Week Randomized, Placebo-Controlled Study
Abstract
Purpose: Androgenetic alopecia (AGA) is the most common type of hair loss in humans, affecting self-esteem and emotional well-being. This study aimed to assess the safety and efficacy of VISPOTM, a standardized saw palmetto oil (2-3% β-sitosterol), in subjects with mild-to-moderate AGA.
Methods: In a double-blind, placebo-controlled, four-arm clinical study, 80 healthy male and female subjects aged 18-50 years were randomly allocated (1:1:1:1) to receive either 400 mg capsules of VISPO or 5 mL of a topical formulation containing 20% VISPO or the respective placebo once daily for 16 weeks. The primary endpoints included hair count (hair comb and hair pull tests) and the self-assessment of perceived efficacy. Objective evaluation was performed using the global photographic assessment score. Hair density, thickness, and anagen/telogen ratio were evaluated using phototrichogram analysis.
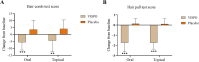
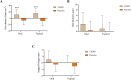
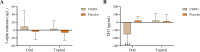
Results: At the end of the study, oral and topical formulations of VISPO reduced hair fall by up to 29% (p<0.001) and 22.19% (p<0.01) from the baseline, respectively. Hair density increased by 5.17% and 7.61% in the oral and topical VISPO groups, respectively (p<0.001). In addition, oral ingestion of VISPO resulted in a marked reduction in serum dihydrotestosterone (DHT) levels in the subjects compared to placebo (p<0.001). However, the effect of the VISPO formulations on the anagen/telogen ratio was insignificant. No serious adverse effects were observed during the study.
Conclusion: VISPO formulations reduced hair fall and promoted hair regrowth and scalp appearance in AGA patients.
Keywords: androgenetic alopecia; fatty acids; hair loss; saw palmetto; β-sitosterol.
© 2023 Sudeep et al.
Conflict of interest statement
The investigational product VISPOTM is a proprietary extract manufactured by Vidya Herbs Pvt., Ltd. Heggar Venkataramana Sudeep, Richards Aleksander, Kuluvar Gouthamchandra, and Kodimule Shyamprasad are employed by Vidya Herbs Pvt. Ltd., and Sriram Rashmi s employed by the BGS Global Institute of Medical Sciences, Bangalore, India. Thomas V. Jestin s employed by Leads Clinical Research and Bio Services Private Ltd. (Bangalore, India). The authors declare no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

