Inhibition of T-cell activity in alopecia areata: recent developments and new directions
- PMID: 38022501
- PMCID: PMC10657858
- DOI: 10.3389/fimmu.2023.1243556
Inhibition of T-cell activity in alopecia areata: recent developments and new directions
Abstract
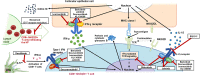
Alopecia areata (AA) is an autoimmune disease that has a complex underlying immunopathogenesis characterized by nonscarring hair loss ranging from small bald patches to complete loss of scalp, face, and/or body hair. Although the etiopathogenesis of AA has not yet been fully characterized, immune privilege collapse at the hair follicle (HF) followed by T-cell receptor recognition of exposed HF autoantigens by autoreactive cytotoxic CD8+ T cells is now understood to play a central role. Few treatment options are available, with the Janus kinase (JAK) 1/2 inhibitor baricitinib (2022) and the selective JAK3/tyrosine kinase expressed in hepatocellular carcinoma (TEC) inhibitor ritlecitinib (2023) being the only US Food and Drug Administration-approved systemic medications thus far for severe AA. Several other treatments are used off-label with limited efficacy and/or suboptimal safety and tolerability. With an increased understanding of the T-cell-mediated autoimmune and inflammatory pathogenesis of AA, additional therapeutic pathways beyond JAK inhibition are currently under investigation for the development of AA therapies. This narrative review presents a detailed overview about the role of T cells and T-cell-signaling pathways in the pathogenesis of AA, with a focus on those pathways targeted by drugs in clinical development for the treatment of AA. A detailed summary of new drugs targeting these pathways with expert commentary on future directions for AA drug development and the importance of targeting multiple T-cell-signaling pathways is also provided in this review.
Keywords: Alopecia areata; JAK inhibitor; T cells; T-cell receptor; autoimmune disease.
Copyright © 2023 Passeron, King, Seneschal, Steinhoff, Jabbari, Ohyama, Tobin, Randhawa, Winkler, Telliez, Martin and Lejeune.
Conflict of interest statement
TP has received honoraria and/or consultation fees from AbbVie, Almirall, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, GSK, Incyte, Janssen, LEO Pharma, MSD, Novartis, Pfizer, Sanofi Genzyme, Sun Pharma, UCB Pharma, and Vyne Therapeutics. BK has received honoraria and/or consultation fees from AbbVie, Aclaris Therapeutics, AltruBio, Almirall, Arena Pharmaceuticals, Bioniz Therapeutics, Bristol Myers Squibb, Concert Pharmaceuticals, Dermavant Sciences, Eli Lilly, Incyte, LEO Pharma, Otsuka/Visterra, Pfizer, Regeneron, Sanofi Genzyme, TWi Biotechnology, and Viela Bio and speakers bureau fees from Pfizer. JS has been an advisor, speaker, or investigator for AbbVie, Calypso Biotech, Eli Lilly, Novartis, Pierre Fabre, and Sanofi Genzyme. MS has received honoraria, consultation fees, or investigator fees from AbbVie, Almirall, Arena, Algorithm, Avon, Baiersdorf, Bayer Health, BMS, Celgene, Chugai, Ducray, Eli Lilly, Galderma, Genentech, GSK, Incyte, Kiniksa, LEO Pharma, L’Oreal, Maruho, Mitsubishi, Janssen, Novartis, Pfizer, Pierre Fabre, Qatar Pharma, Regeneron, Sanofi, Toray, Trevi, Vertex, and ZymoGenetics. AJ has received institutional grants from Arena Pharmaceuticals, InSilico Medicine, and Pfizer and honoraria and/or consultation fees from Pfizer. MO has received lecture fees from Eli Lilly Japan; advisory fees from Eli Lilly Japan, Pfizer Japan Inc., Maruho Co., Bristol Myers Squibb Japan., Taisho Pharmaceutical Co., AbbVie GK, and ROHTO Pharmaceutical Co.; and research grants not related to the submitted work from Maruho Co., Shiseido Co, Advantest Corp., and Sun Pharma Japan Ltd. DT has been an advisor, speaker, or investigator for Pfizer, Frequency Therapeutics, Nacuity Pharmaceuticals, Menarini Group, Galderma, Sanofi Genzyme, and Janssen. SR, AW, J-BT, DM, and AL are employees of Pfizer and hold stock or stock options in Pfizer. The authors declare that this study received funding from Pfizer. The funder had the following involvement in the study: the study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
Figures


References
-
- Mostaghimi A, Gao W, Ray M, Bartolome L, Wang T, Carley C, et al. . Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-sponsored insured population. JAMA Dermatol (2023) 159(4):411–8. doi: 10.1001/jamadermatol.2023.0002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

