Detection of anti-SARS-CoV-2 salivary antibodies in vaccinated adults
- PMID: 38022522
- PMCID: PMC10661372
- DOI: 10.3389/fimmu.2023.1296603
Detection of anti-SARS-CoV-2 salivary antibodies in vaccinated adults
Abstract
Since the introduction of efficient anti-SARS-CoV-2 vaccines, the detection of antibodies becomes useful for immunological monitoring and COVID-19 control. Therefore, this longitudinal study aimed to evaluate the detection of SARS-CoV-2 antibodies in the serum and saliva of COVID-19-vaccinated adults. The study included 13 not vaccinated and 35 vaccinated participants with two doses of CoronaVac (Sinovac/Butantan) vaccine who subsequently received BNT162b2 (Pfizer-BioNTech) vaccine as a booster dose. Vaccinated participants donated saliva and serum in three different time points. Enzyme-linked immunosorbent assay was used for antibody detection. In our results, the serum neutralizing antibodies (NAb) were detected in 34/35 samples after second dose and in 35/35 samples one and five months after the booster dose. In saliva, NAb were detected in 30/35 samples after second dose and in 35/35 of samples one and five months after the booster dose. IgA was detected in 19/34 saliva samples after second dose, in 18/35 one month after the booster and in 30/35 five months after. IgG in saliva was detected in 1/34 samples after second dose, 33/35 samples one month after the booster dose and in 20/35 five months after. A strong correlation was found between IgG and neutralizing activity in saliva, and salivary IgA would be a sign of recent exposure to the virus. In conclusion, saliva can be suitable for monitoring antibodies anti-SARS-CoV-2 after vaccination. Heterologous vaccination contributed to increase anti-SARS-CoV-2 antibodies in the Brazilian health context. Complementary studies with large groups are mandatory to conclude the interest in following mucosal immunity.
Keywords: COVID-19; COVID-19 vaccines; IgA; IgG; SARS-CoV-2; antibodies; neutralizing antibodies; saliva.
Copyright © 2023 Castro, Chardin, Amorim dos Santos, Barra, Castilho, Souza, Magalhães, Acevedo and Guerra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
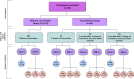
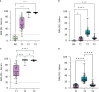
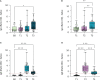
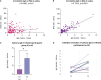
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

