Ligand-based targeting of c-kit using engineered γδ T cells as a strategy for treating acute myeloid leukemia
- PMID: 38022523
- PMCID: PMC10679681
- DOI: 10.3389/fimmu.2023.1294555
Ligand-based targeting of c-kit using engineered γδ T cells as a strategy for treating acute myeloid leukemia
Abstract
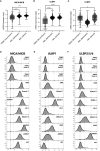
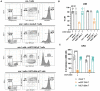
The application of immunotherapies such as chimeric antigen receptor (CAR) T therapy or bi-specific T cell engager (BiTE) therapy to manage myeloid malignancies has proven more challenging than for B-cell malignancies. This is attributed to a shortage of leukemia-specific cell-surface antigens that distinguish healthy from malignant myeloid populations, and the inability to manage myeloid depletion unlike B-cell aplasia. Therefore, the development of targeted therapeutics for myeloid malignancies, such as acute myeloid leukemia (AML), requires new approaches. Herein, we developed a ligand-based CAR and secreted bi-specific T cell engager (sBite) to target c-kit using its cognate ligand, stem cell factor (SCF). c-kit is highly expressed on AML blasts and correlates with resistance to chemotherapy and poor prognosis, making it an ideal candidate for which to develop targeted therapeutics. We utilize γδ T cells as a cytotoxic alternative to αβ T cells and a transient transfection system as both a safety precaution and switch to remove alloreactive modified cells that may hinder successful transplant. Additionally, the use of γδ T cells permits its use as an allogeneic, off-the-shelf therapeutic. To this end, we show mSCF CAR- and hSCF sBite-modified γδ T cells are proficient in killing c-kit+ AML cell lines and sca-1+ murine bone marrow cells in vitro. In vivo, hSCF sBite-modified γδ T cells moderately extend survival of NSG mice engrafted with disseminated AML, but therapeutic efficacy is limited by lack of γδ T-cell homing to murine bone marrow. Together, these data demonstrate preclinical efficacy and support further investigation of SCF-based γδ T-cell therapeutics for the treatment of myeloid malignancies.
Keywords: acute myeloid leukemia (AML); c-kit (CD117); chimeric antigen receptor (CAR); gamma delta (γδ) T cells; ligand-based therapeutics; secreted bispecific T cell engager; stem cell factor (SCF).
Copyright © 2023 Branella, Lee, Okalova, Parwani, Alexander, Arthuzo, Fedanov, Yu, McCarty, Brown, Chandrakasan, Petrich, Doering and Spencer.
Conflict of interest statement
BY, HB, DM, and BP are employees of Expression Therapeutics, which develops cancer immunotherapies using engineered γδ T cells. GB, CD, and HS are inventors on a patent application describing ligand-based cell and gene therapies for hematopoietic cancers owned by Emory University and licensed to Expression Therapeutics, Inc. HS and CD are cofounders of Expression Therapeutics and own equity in the company. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict-of-interest policies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. . Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (Transcend nhl 001): A multicentre seamless design study. Lancet (2020) 396(10254):839–52. doi: 10.1016/s0140-6736(20)31366-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

