SARS-CoV-2 specific immune responses in overweight and obese COVID-19 patients
- PMID: 38022529
- PMCID: PMC10653322
- DOI: 10.3389/fimmu.2023.1287388
SARS-CoV-2 specific immune responses in overweight and obese COVID-19 patients
Abstract
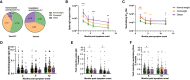
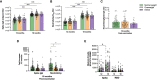
Obesity is a known risk factor for severe respiratory tract infections. In this prospective study, we assessed the impact of being obese or overweight on longitudinal SARS-CoV-2 humoral and cellular responses up to 18 months after infection. 274 patients provided blood samples at regular time intervals up to 18 months including obese (BMI ≥30, n=32), overweight (BMI 25-29.9, n=103) and normal body weight (BMI 18.5-24.9, n=134) SARS-CoV-2 patients. We determined SARS-CoV-2 spike-specific IgG, IgA, IgM levels by ELISA and neutralising antibody titres by neutralisation assay. RBD- and spike-specific memory B cells were investigated by ELISpot, spike- and non-spike-specific IFN-γ, IL-2 and IFN-γ/IL-2 secreting T cells by FluoroSpot and T cell receptor (TCR) sequencing was performed. Higher BMI correlated with increased COVID-19 severity. Humoral and cellular responses were stronger in overweight and obese patients than normal weight patients and associated with higher spike-specific IgG binding titres relative to neutralising antibody titres. Linear regression models demonstrated that BMI, age and COVID-19 severity correlated independently with higher SARS-CoV-2 immune responses. We found an increased proportion of unique SARS-CoV-2 specific T cell clonotypes after infection in overweight and obese patients. COVID-19 vaccination boosted humoral and cellular responses irrespective of BMI, although stronger immune boosting was observed in normal weight patients. Overall, our results highlight more severe disease and an over-reactivity of the immune system in overweight and obese patients after SARS-CoV-2 infection, underscoring the importance of recognizing overweight/obese individuals as a risk group for prioritisation for COVID-19 vaccination.
Keywords: COVID-19; TCR; cellular; neutralising; obesity; overweight; spike; vaccination.
Copyright © 2023 Onyango, Zhou, Bredholt, Brokstad, Lartey, Mohn, Özgümüs, Kittang, Linchausen, Shafiani, Elyanow, Blomberg, Langeland, Cox and Bergen COVID-19 Research Group.
Conflict of interest statement
Authors SS and RE are employed by Adaptive Biotechnologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O'Rahilly S, Aveyard P, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol (2021) 9(6):350–9. doi: 10.1016/S2213-8587(21)00089-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

