Niacinamide enhances cathelicidin mediated SARS-CoV-2 membrane disruption
- PMID: 38022563
- PMCID: PMC10663372
- DOI: 10.3389/fimmu.2023.1255478
Niacinamide enhances cathelicidin mediated SARS-CoV-2 membrane disruption
Abstract
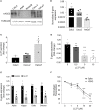
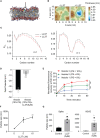
The continual emergence of SARS-CoV-2 variants threatens to compromise the effectiveness of worldwide vaccination programs, and highlights the need for complementary strategies for a sustainable containment plan. An effective approach is to mobilize the body's own antimicrobial peptides (AMPs), to combat SARS-CoV-2 infection and propagation. We have found that human cathelicidin (LL37), an AMP found at epithelial barriers as well as in various bodily fluids, has the capacity to neutralise multiple strains of SARS-CoV-2. Biophysical and computational studies indicate that LL37's mechanism of action is through the disruption of the viral membrane. This antiviral activity of LL37 is enhanced by the hydrotropic action of niacinamide, which may increase the bioavailability of the AMP. Interestingly, we observed an inverse correlation between LL37 levels and disease severity of COVID-19 positive patients, suggesting enhancement of AMP response as a potential therapeutic avenue to mitigate disease severity. The combination of niacinamide and LL37 is a potent antiviral formulation that targets viral membranes of various variants and can be an effective strategy to overcome vaccine escape.
Keywords: COVID-19; LL37; SARS-CoV-2; antimicrobial peptides; niacinamide; skin immunity; viral membrane.
Copyright © 2023 Bhatt, Dam, Khedkar, Lall, Pandey, Kataria, Ajnabi, Gulzar, Dias, Waskar, Raut, Sundaramurthy, Vemula, Ghatlia, Majumdar and Jamora.
Conflict of interest statement
PD, JR, MW, NG, and AM are employees of Unilever R&D. IFOM - The AIRC Institute of Molecular Oncology Italy, inStem- Institute for Stem Cell Science and Regenerative Medicine India, and Unilever R&D have filed patent applications for AMP & niacinamide antiviral combination on which TB, CJ, NG, and AM are listed as inventors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

