A phase I oncolytic virus trial with vesicular stomatitis virus expressing human interferon beta and tyrosinase related protein 1 administered intratumorally and intravenously in uveal melanoma: safety, efficacy, and T cell responses
- PMID: 38022659
- PMCID: PMC10644866
- DOI: 10.3389/fimmu.2023.1279387
A phase I oncolytic virus trial with vesicular stomatitis virus expressing human interferon beta and tyrosinase related protein 1 administered intratumorally and intravenously in uveal melanoma: safety, efficacy, and T cell responses
Abstract
Introduction: Metastatic uveal melanoma (MUM) has a poor prognosis and treatment options are limited. These patients do not typically experience durable responses to immune checkpoint inhibitors (ICIs). Oncolytic viruses (OV) represent a novel approach to immunotherapy for patients with MUM.
Methods: We developed an OV with a Vesicular Stomatitis Virus (VSV) vector modified to express interferon-beta (IFN-β) and Tyrosinase Related Protein 1 (TYRP1) (VSV-IFNβ-TYRP1), and conducted a Phase 1 clinical trial with a 3 + 3 design in patients with MUM. VSV-IFNβ-TYRP1 was injected into a liver metastasis, then administered on the same day as a single intravenous (IV) infusion. The primary objective was safety. Efficacy was a secondary objective.
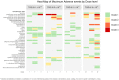
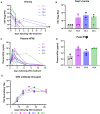
Results: 12 patients with previously treated MUM were enrolled. Median follow up was 19.1 months. 4 dose levels (DLs) were evaluated. One patient at DL4 experienced dose limiting toxicities (DLTs), including decreased platelet count (grade 3), increased aspartate aminotransferase (AST), and cytokine release syndrome (CRS). 4 patients had stable disease (SD) and 8 patients had progressive disease (PD). Interferon gamma (IFNγ) ELIspot data showed that more patients developed a T cell response to virus encoded TYRP1 at higher DLs, and a subset of patients also had a response to other melanoma antigens, including gp100, suggesting epitope spreading. 3 of the patients who responded to additional melanoma antigens were next treated with ICIs, and 2 of these patients experienced durable responses.
Discussion: Our study found that VSV-IFNβ -TYRP1 can be safely administered via intratumoral (IT) and IV routes in a previously treated population of patients with MUM. Although there were no clear objective radiographic responses to VSV-IFNβ-TYRP1, dose-dependent immunogenicity to TYRP1 and other melanoma antigens was seen.
Keywords: epitope spreading; immunotherapy; oncolytic virus; phase 1; uveal melanoma.
Copyright © 2023 Smith, Peng, Pulido, Weisbrod, Strand, Allred, Newsom, Zhang, Packiriswamy, Kottke, Tonne, Moore, Montane, Kottschade, McWilliams, Dudek, Yan, Dimou, Markovic, Federspiel, Vile, Dronca and Block.
Conflict of interest statement
KP: Imanis Life Sciences stock; Vyriad stock. RM: Zentalis pharmaceuticals consulting. AD: Guardant health advisory board; TP therapeutics advisory board; Novartis clinical trial support; Sorrento therapeutics clinical trial support; Syntrix therapeutics clinical trial support; AnHeart Therapeutics clinical trial support; Merck clinical trial support; Intellisphere LLC honoraria. NP: Imanis life sciences LLCprincipal scientist. LK: Immunocore consulting; Novartis consulting. MS: Bristol-Myers Squibb grant/contract; Sorrento therapeutics grant/contract; other intellectual property. RV: Oncolytics Biotech, Canada scientific advisory board; Greenfire/MGFB scientific advisory board. RD: Bristol Myers Squibb funding; Elsai consulting; Elsevier advisory board; EMD Serono Inc consulting; Genzyme Corporation case series discussion; Immunovaccine Technologies consulting; Natera advisory board; Regeneron Pharmaceuticals consultant; Sanofi Genzyme advisory board. MB: Alkermes grant/contract; Bristol-Meyers Squibb grant/contract; Genentech grant/contract; Immune Design grant/contract; Marker Therapeutics grant/ contract; Merck grant/contract; nFerence grant/contract; Pharmacyclics grant/contract; TILT Biotherapeutics grant/contract; Transgene grant/contract; Viewpoint MolecularTargeting grant/contract. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission.
Figures


References
-
- SEER*Explorer . Melanoma of the skin, Recent Trends in SEER Relative Survival Rate . Available at: https://seer.cancer.gov/statistics-network/explorer/application.html?sit... (Accessed September 16 2022).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

