Fluorescence-readout as a powerful macromolecular characterisation tool
- PMID: 38023522
- PMCID: PMC10664555
- DOI: 10.1039/d3sc04052f
Fluorescence-readout as a powerful macromolecular characterisation tool
Abstract
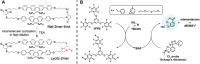
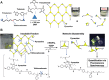
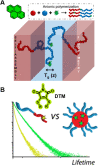
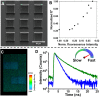
The last few decades have witnessed significant progress in synthetic macromolecular chemistry, which can provide access to diverse macromolecules with varying structural complexities, topology and functionalities, bringing us closer to the aim of controlling soft matter material properties with molecular precision. To reach this goal, the development of advanced analytical techniques, allowing for micro-, molecular level and real-time investigation, is essential. Due to their appealing features, including high sensitivity, large contrast, fast and real-time response, as well as non-invasive characteristics, fluorescence-based techniques have emerged as a powerful tool for macromolecular characterisation to provide detailed information and give new and deep insights beyond those offered by commonly applied analytical methods. Herein, we critically examine how fluorescence phenomena, principles and techniques can be effectively exploited to characterise macromolecules and soft matter materials and to further unravel their constitution, by highlighting representative examples of recent advances across major areas of polymer and materials science, ranging from polymer molecular weight and conversion, architecture, conformation to polymer self-assembly to surfaces, gels and 3D printing. Finally, we discuss the opportunities for fluorescence-readout to further advance the development of macromolecules, leading to the design of polymers and soft matter materials with pre-determined and adaptable properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
















References
-
- Xu J. Macromolecules. 2019;52:9068–9093. doi: 10.1021/acs.macromol.9b01365. - DOI
-
- Espeel P. Du Prez F. E. Macromolecules. 2015;48:2–14. doi: 10.1021/ma501386v. - DOI
-
- Corrigan N. Jung K. Moad G. Hawker C. J. Matyjaszewski K. Boyer C. Prog. Polym. Sci. 2020;111:101311. doi: 10.1016/j.progpolymsci.2020.101311. - DOI
Publication types
LinkOut - more resources
Full Text Sources

