Efficacy of Messenger RNA-1273 Against Severe Acute Respiratory Syndrome Coronavirus 2 Acquisition in Young Adults From March to December 2021
- PMID: 38023544
- PMCID: PMC10655942
- DOI: 10.1093/ofid/ofad511
Efficacy of Messenger RNA-1273 Against Severe Acute Respiratory Syndrome Coronavirus 2 Acquisition in Young Adults From March to December 2021
Abstract
Background: The efficacy of messenger RNA (mRNA)-1273 against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is not well defined, particularly among young adults.
Methods: Adults aged 18-29 years with no known history of SARS-CoV-2 infection or prior vaccination for coronavirus disease 2019 (COVID-19) were recruited from 44 US sites from 24 March to 13 September 2021 and randomized 1:1 to immediate vaccination (receipt of 2 doses of mRNA-1273 vaccine at months 0 and 1) or the standard of care (receipt of COVID-19 vaccine). Randomized participants were followed up for SARS-CoV-2 infection measured by nasal swab testing and symptomatic COVID-19 measured by nasal swab testing plus symptom assessment and assessed for the primary efficacy outcome. A vaccine-declined observational group was also recruited from 16 June to 8 November 2021 and followed up for SARS-CoV-2 infection as specified for the randomized participants.
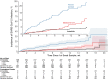
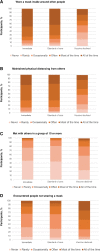
Results: The study enrolled 1149 in the randomized arms and 311 in the vaccine-declined group and collected >122 000 nasal swab samples. Based on randomized participants, the efficacy of 2 doses of mRNA-1273 vaccine against SARS-CoV-2 infection was 52.6% (95% confidence interval, -14.1% to 80.3%), with the majority of infections due to the Delta variant. Vaccine efficacy against symptomatic COVID-19 was 71.0% (95% confidence interval, -9.5% to 92.3%). Precision was limited owing to curtailed study enrollment and off-study vaccination censoring. The incidence of SARS-CoV-2 infection in the vaccine-declined group was 1.8 times higher than in the standard-of-care group.
Conclusions: mRNA-1273 vaccination reduced the incidence of SARS-CoV-2 infection from March to September 2021, but vaccination was only one factor influencing risk.
Clinical trials registration: NCT04811664.
Keywords: COVID-19; SARS-CoV-2 infection; lifestyle circumstances; mRNA-1273 vaccine.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures

References
-
- ModernaTX I. Vaccine information fact sheet for recipients and caregivers. Available at: https://www.fda.gov/media/144638/download. Accessed 3 October 2022.
-
- US Food & Drug Administration . Emergency use authorization for Moderna’s COVID-19 vaccine. Available at: https://eua.modernatx.com/covid19vaccine-eua/fda-letter-eua.pdf. Accessed 8 July 2022.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

