Reduction in Depressive Symptoms in People who Inject Drugs who Are Cured of Hepatitis C Virus Infection: The HERO Study
- PMID: 38023556
- PMCID: PMC10644781
- DOI: 10.1093/ofid/ofad498
Reduction in Depressive Symptoms in People who Inject Drugs who Are Cured of Hepatitis C Virus Infection: The HERO Study
Abstract
Background: Depressive symptoms are prevalent among people who inject drugs (PWID) and people with hepatitis C virus (HCV). We examined changes in depressive symptoms among HCV-infected PWID following direct-acting antiviral treatments to evaluate whether these changes differed by history of depressive symptoms, substance use, or HCV treatment outcome.
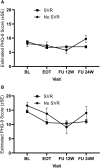
Methods: We conducted a secondary analysis of the HERO Study (NCT02824640), a pragmatic randomized clinical trial among PWID, to test the effectiveness of HCV care models. Depressive symptoms (primary outcome) were measured using the Patient Health Questionnaire (PHQ-9) at baseline, end of treatment (EOT), and at follow-up 12 and 24 weeks after EOT. Sustained virologic response (SVR) was defined as undetectable HCV RNA at ≥12 weeks following EOT. Baseline drug use was defined as having a positive urine screening test for amphetamine, methamphetamine, benzodiazepine, cocaine, cannabis, opiate, or oxycodone.
Results: The sample (n = 498) was 72.3% male, 64.2% White, and on average 43.9 years old. In patients who achieved SVR (F(3432) = 4.58; P = .004) and those with drug use at baseline (F(3478) = 5.11; P < .01), PHQ-9 scores significantly declined over time, with scores lower at EOT and both follow-ups as compared with baseline. Mean PHQ-9 scores at EOT and follow-ups were significantly lower than at baseline, except for those with no depression or mild depression at baseline.
Conclusions: This study showed that HCV treatment in PWID is associated with sustained declines in depression up to 24 weeks post-treatment among those who achieve SVR and that drug use does not interfere with improvement in depressive symptoms.
Keywords: DAA medication; HCV; PWID; depression.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J.F. has received research grant support from Gilead Sciences. A.Y.K. has served on advisory boards for Biomarin. A.F.L. has received research grant support from Gilead and Merck. The Task Force for Global Health receives funds for the general support of the Coalition for Global Hepatitis Elimination from Abbott, Gilead, AbbVie, Merck, Siemens, Roche, Pharco, Zydus-Cadila, governmental agencies, and philanthropic organizations. A.H.L. has served on advisory boards for Gilead Sciences and Merck Pharmaceuticals and received research funding from Gilead Sciences. S.H.M. has received speaker fees from Gilead Sciences. All other authors declare no competing interests.
Figures

References
-
- Colledge S, Larney S, Peacock A, et al. Depression, post-traumatic stress disorder, suicidality and self-harm among people who inject drugs: a systematic review and meta-analysis. Drug Alcohol Depend 2020; 207:107793. - PubMed
-
- Grebely J, Dore GJ, Alami NN, et al. Safety and efficacy of glecaprevir/pibrentasvir in patients with chronic hepatitis C genotypes 1–6 receiving opioid substitution therapy. Int J Drug Policy 2019; 66:73–9. - PubMed
-
- Fortier E, Alavi M, Bruneau J, et al. Depression, anxiety, and stress among people with chronic hepatitis C virus infection and a history of injecting drug use in New South Wales, Australia. J Addict Med 2017; 11:10–8. - PubMed

