Immune senescence in aged APP/PS1 mice
- PMID: 38023614
- PMCID: PMC10659760
- DOI: 10.1515/nipt-2023-0015
Immune senescence in aged APP/PS1 mice
Abstract
Objectives: To evaluate the linkage between age and deficits in innate and adaptive immunity which heralds both Alzheimer's disease (AD) onset and progression. The pathobiological events which underlie and tie these outcomes remain not fully understood.
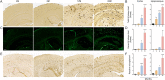
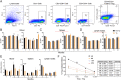
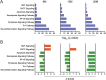
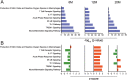
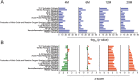
Methods: To investigate age-dependent immunity in AD, we evaluated innate and adaptive immunity in coordinate studies of regulatory T cell (Treg) function, T cell frequencies, and microglial integrity. These were assessed in blood, peripheral lymphoid tissues, and the hippocampus of transgenic (Tg) amyloid precursor protein/presenilin 1 (APP/PS1) against non-Tg mice. Additionally, immune arrays of hippocampal tissue were performed at 4, 6, 12, and 20 months of age.
Results: APP/PS1 mice showed progressive impairment of Treg immunosuppressive function with age. There was partial restoration of Treg function in 20-month-old mice. Ingenuity pathway analyses of hippocampal tissues were enriched in inflammatory, oxidative, and cellular activation pathways that paralleled advancing age and AD-pathobiology. Operative genes in those pathways included, but were not limited to triggering receptor on myeloid cells 1 (TREM1), T helper type 1 (Th1), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways. Interleukin-17 (IL-17), nitric oxide, acute phase, and T cell receptor signaling pathways were also perturbed. Significant inflammation was observed at 6- and 12-months. However, at 20-months, age associated partial restoration of Treg function reduced inflammatory phenotype.
Conclusions: Impaired Treg function, inflammation and oxidative stress were associated with AD pathology. Age associated partial restoration of Treg function in old mice reduced the hippocampal inflammatory phenotype. Restoring Treg suppressive function can be a therapeutic modality for AD.
Keywords: Alzheimer’s disease; Treg; aging; neuroinflammation; oxidative stress.
© 2023 the author(s), published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Competing interests: No conflicts of interest.
Figures
References
-
- Vitale P, Salgueiro-Pereira AR, Lupascu CA, Willem M, Migliore R, Migliore M, et al. Analysis of age-dependent alterations in excitability properties of CA1 pyramidal neurons in an APPPS1 model of Alzheimer’s disease. Front Aging Neurosci. 2021;13:668948. doi: 10.3389/fnagi.2021.668948. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
